STERILIZATION
Tubal Sterilization
This is a surgical method of preventing the ovum from being fertilized or reaching the uterine cavity by occluding or otherwise interrupting the fallopian tubes. This method is effective and permanent. There is an associated decreased risk of PID and ovarian cancer in women who have undergone sterilization procedures.12 Drawbacks to this method include the risks inherent to any surgical procedure as well as the permanence of the procedure. Sterilization regret is highly correlated with age younger than 30 and unpredictable life events such as change in marital status or death of a child.12 Reversal is costly and unpredictable. Also, there is an increased risk for ectopic pregnancy, should pregnancy occur.
Vasectomy
Vasectomy is a surgical method of preventing the release of sperm in the ejaculate by disrupting the vas deferens typically with ligation and cautery. This method is less expensive than tubal ligation; restoring fertility after vasectomy is unreliable and expensive. Another method should be used until documentation of azoospermia, which may be about 10 weeks. Bleeding and infection are rare complications.13
EMERGENCY CONTRACEPTION
This term often refers to high-dose progestin in pill form used postcoitally to prevent pregnancy by preventing ovulation, altering cervical mucus, or preventing implantation of fertilized ovum. Progestin pills do not interrupt an already-implanted pregnancy, and is not teratogenic.3 The copper IUD may also be used as emergency contraception, as well as the antiprogestin drug ulipristal. The copper IUD is the most effective form of postcoital contraception, with the additional benefit of remaining in place to provide ongoing protection.14
• Ella. 30 mg ulipristal is taken as soon as possible, but within 120 hours of intercourse.14
• Plan B. 75 μg levonorgestrel is taken within 72 hours of intercourse, with second 75 μg tablet taken 12 hours later.3,13 Some sources say the first tablet may actually be given within 120 hours of intercourse.3 Plan B is now readily available over the counter.
• Alternatively, combined OCPs may be used. The first dose should be given within the first 72 hours after unprotected intercourse, and the second dose given 12 hours after the first dose. Many brand name OCPs can be used for emergency contraception including:
• Preven Kit. Two pills per dose (0.5 mg of levonorgestrel and 100 μg of ethinyl estradiol per dose)
• Ovral. Two pills per dose (0.5 mg of levonorgestrel and 100 μg of ethinyl estradiol per dose)
• Lo/Ovral. Four pills per dose (0.6 mg of levonorgestrel and 120 μg of ethinyl estradiol per dose)
• Nordette. Four pills per dose (0.6 mg of levonorgestrel and 120 μg of ethinyl estradiol per dose)
• Triphasil. Four pills per dose (0.5 mg of levonorgestrel and 120 μg of ethinyl estradiol per dose)
• Ovrette. 20 pills per dose (1.5 mg of levonorgestrel per dose)
• Seasonale. Four pills per dose (0.6 mg of levonorgestrel and 120 μg of ethinyl estradiol per dose)
• Alesse. Five pills per dose (0.5 mg of levonorgestrel and 100 μg of ethinyl estradiol per dose)3,13
REFERENCES
1. Samra O. Contraception (online). http://www.emedicine.com. Accessed on April 4, 2014.
2. Zieman M, Hatcher R. Managing contraception. New York, NY: Ardent Media; 2012.
3. Steiner MJ, Dominik R, Rountree RW, et al. Contraceptive effectiveness of a polyurethane condom and a latex condom: a randomized controlled trial. Obstet Gynecol 2003;101:539–547.
4. Dean G, Goldberg A. Intrauterine contraception (IUD): Overview. www.uptodate.com. Accessed on April 4, 2014.
5. Johnson BA. Insertion and removal of intrauterine devices. Am Fam Physician 2005;71:95–102.
6. Herndon EJ. New contraceptive options. Am Fam Physician 2004;69:853–860.
7. CerelSuhl SL, Yeager BF. Update on oral contraceptives. Am Fam Physician 1999;60(7):2073–2084.
8. Martin KA, Barbieri AR. Overview of the use of estrogen progestin contraceptives. http://www.uptodate.com. Accessed on April 4, 2014.
9. Apgar BA, Greenberg G. Using progestins in clinical practice. Am Fam Physician 2000;62(8)1839–1846, 1849–1850.
10. Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril 2009;91:1646.
11. Kaunitz A. Depot medroxyprogesterone acetate for contraception. www.uptodate.com. Accessed on April 4, 2014.
12. Baill IC, Cullins VE, Sangeeta P. Counseling issues in tubal sterilization. Am Fam Physician 2003;67:1287–1294, 1301–1302.
13. Dassow P, Bennett J. Vasectomy: an update. Am Fam Physician 2006;74:2069–2074.
14. Bosworth MC, Okesola PL, Low SB. An update on emergency contraception. Am Fam Physician 2014;89:545–550.
|
GENERAL PRINCIPLES
The diagnosis of infertility is established after 1 year of regular unprotected intercourse in which a pregnancy has not been achieved. In women older than 35, infertility is diagnosed after 6 months of regular unprotected intercourse. Fecundity is the probability of achieving pregnancy in one menstrual cycle; approximately 50% of couples are able to conceive after 3 months, 75% after 6 months, and 90% after 1 year. In the Unites States, 12% of reproductive age women are affected by infertility; this proportion increases with the age of the female partner.1–4
There are many causes of infertility, including abnormalities of any portion of the male or female reproductive system. Infertility is due to a single cause in the majority of couples, but more than one factor may contribute to infertility. Therefore, a comprehensive history, physical examination, and diagnostic evaluation are recommended for all couples.5
Common Etiology and Pathophysiology
• Male factors. Male cause for infertility occurs in 25% of couples. The most common male etiologic factor is a varicocele. Other causative factors include low or absent sperm count, oligospermia or azoospermia, disorders of sperm function or motility, asthenospermia, and abnormalities of sperm morphology, teratospermia. Autoimmunity as a cause of male infertility is rare.4
• Ovulatory dysfunction. Disorders of ovulation account for approximately 27% of cases of infertility.6 The possible causes may be grouped into four major categories:
• Hypothalamic anovulation includes anatomical defects, congenital defects, psychologic trauma, anorexia nervosa, and pharmacologic agents.
• Ovarian anovulation includes ovarian tumors, premature ovarian failure, ovarian dysgenesis, thyroid disease, and adrenal disease.
• Pituitary anovulation includes pituitary tumors and ischemia.
• Integrative anovulation includes nonpsychogenic weight disturbances and polycystic ovarian syndrome (PCOS).
• Tubal. Infertility due to tubal structural damage or adnexal adhesions accounts for approximately 22% of cases of infertility.6 Tubal obstruction may result from previous episodes of salpingitis, although many cases of tubal occlusion are encountered in which no episodes of salpingitis are recalled by the patient. Endometriosis may result in the anatomical distortion of adnexal structures.
• Endometriosis. The chronic inflammation associated with endometriosis may disrupt normal conception by interfering with ovum capture and gamete and embryo transport, or by causing tubal damage. Endometriosis is the cause of approximately 5% of infertility cases.6
• Unexplained infertility. No specific etiologic factor is identified in approximately 17% of infertile couples after an initial diagnostic survey.6
DIAGNOSTIC EVALUATION
A thorough diagnostic survey of both partners is necessary to evaluate all areas of the reproductive system. The workup is begun for women under 35 years old after 12 months of infertility and after 6 months of infertility in women over 35 years old. A meeting with the couple early in the evaluation provides an opportunity to review reproductive biology, discuss the rationale for subsequent tests, and assess the couple’s coping skills.
History
The initial assessment of the couple consists of a thorough history of each partner, taken individually, to assess current and past contributing symptoms, illness, medication, or surgery. The key elements of such a history are outlined in Table 14.2-1.4
Physical Examination
As with the history, a thorough physical examination of each partner is essential. Areas of special attention for each physical are listed in Table 14.2-2.4
Key Areas of Infertility History |
Marriage
Duration of infertility
Fertility in previous relationship
Sexual techniques
Frequency of intercourse (optimal is daily around time of ovulation)
Use of coital lubricants (often spermicidal)
Adult illness
Acute viral or febrile illness in past 3 mo
Orchitis
Renal disease
Sexually transmitted diseases
Tuberculosis
Occupation and habits
Exposure to radiation, chemicals, excessive heat (e.g., hot tub)
Childhood
Cryptorchidism
Age at puberty
Surgery
Herniorrhaphy
Retroperitoneal surgery
Vasectomy
Past medical history
Focus on endocrine conditions
Gynecologic history (Pap smear testing result, treatment)
Contraceptive use
Diethylstilbestrol (DES) use by mother
Douches and lubricant use
Menarche
Menses (regularity and flow)
Mittelschmerz
Drug use
Alcohol, tobacco, excessive caffeine, and other drugs
Anabolic steroids, nitrofurantoin, cimetidine
Physical Examination in Infertility: Areas of Special Attention |
Male | Female |
Hair pattern | Breast formation and galactorrhea |
Genitalia | Distribution of body fat |
Meatus size and location | Hair pattern (virilization) |
Prostate and seminal vesicles | Neurologic |
Scrotum | Anosmia |
Testicular size (>4 cm in long axis) | Visual fields |
Varicocele (standing and with Valsalva maneuver) | Pelvis External genitalia |
Neurologic | Retrovaginal area (endometriosis) |
Anosmia | Uterus and adnexa |
Visual fields | Vagina and cervix |
Laboratory and Diagnostic Testing in Infertility |
Routine laboratory tests
Male
CBC
Semen analysis (at least 2, 4 wk apart)
Urinalysis
Female
CBC
Pap smear
Urinalysis
Special circumstances
Anovulation: serum prolactin and TSH
Galactorrhea: serum prolactin and TSH
Hyperandrogenism: serum prolactin, TSH, LH, FSH, DHEA-S, 17-OH progesterone
Advanced maternal age >35 yr/o: cycle day 2 FSH and estradiol. Indicates decreased ovarian reserve if FSH >10.–20 mIU/mL. If estradiol elevated, >70 pg/mL cannot accurately interpret FSH results.
Laboratory Studies
Each couple is evaluated with a few routine laboratory and appropriately timed studies to assess every major reproductive factor that may contribute to infertility. This comprehensive diagnostic survey can and should be completed for the majority of couples in 3 to 6 months. The evaluation should be individualized on the basis of findings of the history and physical examination, but an initial survey of all major reproductive factors is necessary in all couples and can be coordinated by the family physician. The specifically timed diagnostic tests required for an infertility survey are outlined in Table 14.2-3.4
• Male factors. The male is evaluated with a complete blood count, urinalysis, and at least two semen analyses 4 weeks apart. Each semen analysis is performed on a fresh (within 2 hours), warm specimen obtained by masturbation after at least 2 days of abstinence. Normal results vary between laboratories, but in general include a volume (2 to 5 mL), complete liquefaction within 30 minutes, sperm count (>20 million per mL), sperm motility (>50%), and morphology (WHO >30% normal forms, Kruger Strict Criteria >14%). Evidence of oligospermia after two or more semen analyses requires further evaluation, including blood levels for luteinizing hormone, follicle-stimulating hormone (FSH), and testosterone. Etiology of semen abnormalities is outlined in Table 14.2-4.4,7,8
• Ovulatory dysfunction. Anovulation or inconsistent ovulation may be suggested by history (irregular menses, amenorrhea), and confirmed by an abnormally low-serum progesterone levels in the midluteal phase, or persistently negative home luteinizing hormone (LH) testing. If the patient is not ovulating, further laboratory evaluation is needed (see Table 14.2-3, Special circumstances). Those patients with a diminished ovarian reserve (FSH >10 mIU per mL) should be referred to an infertility specialist.4
• Tubal factors. The female partner must undergo an evaluation for tubal patency. A hysterosalpingogram is obtained if the history and physical examination show no evidence of tubal damage. Otherwise, the patient is referred for laparoscopy.4
TREATMENT
Treatment should not be initiated until the diagnostic survey is complete and the infertility cause or causes identified. The diagnosis should be shared with the couple together and the treatment options outlined. The workup, diagnosis, and treatment of infertility can precipitate intense emotional reactions. The physician should assist the couple in the development of mutual support. Periodic meetings with the couple to review diagnostic or treatment progress provide further opportunity to reinforce coping skills. Referral to self-help groups, such as RESOLVE, Inc. (www.resolve.org), assists the couple in broadening their support systems.
• Male factors. Consultation with urology is necessary to coordinate treatment for a varicocele or other causes of sperm dysfunction.
• Ovulatory dysfunction. Treatment with clomiphene should be considered for women diagnosed with anovulation. Amenorrheic and oligomenorrheic women attempting to conceive are among the most suitable patients for clomiphene. Patients with other cause for their anovulation respond best to specific therapy, such as surgery for a pituitary tumor or medical therapy for thyroid disease. Obese women with insulin insensitivity and/or PCOS may benefit from metformin therapy initiated at 500 mg PO daily and titrated up over several weeks, with typical dosing of 1,500 mg daily. It is generally a preliminary option to clomiphene in women with obesity and insulin insensitivity.7
Clomiphene citrate is first-line pharmacotherapy for women with anovulatory causes of infertility. The starting dose for clomiphene citrate is 50 mg PO daily on menstrual cycle days 3 to 7. Ovulation should be expected 3 to 8 days after the treatment ends and should be confirmed by LH home detection kit and an elevated serum progesterone on day 21 (approximately 2 weeks after the last clomiphene dose). If midluteal progesterone is greater than 10 pg per mL, continue the same clomiphene dose on subsequent cycles. If the midluteal progesterone level is less than 10 pg per mL, the dose of clomiphene can be increased by 50 mg on subsequent cycles until the patient is ovulating. Most women should begin ovulation at 50 mg dosing. It is unusual to require more than 150 mg, maximum dose 250 mg, to achieve ovulation. At this point, adjunctive therapy with metformin or switching therapy may be considered. The patient should be aware of the common side effects of clomiphene therapy: ovarian enlargement (13.9% of cases), vasomotor flushes (10.7%), abdominal or pelvic discomfort (7.4%), and multiple gestation (<5% and usually twinning). If ovulation does not occur despite clomiphene therapy, consultation with an infertility specialist is recommended.3,7
Etiologies of Semen Abnormalities |
Count abnormality |
|
Oligozoospermia | Endocrinopathies (androgen receptor defect) Varicocele Maturation arrest Hypospermatogenesis |
Azoospermia | Klinefelter syndrome Sertoli-cell–only syndrome Seminiferous tubule or Leydig cell failure Hypogonadotropic hypogonadism Ductal obstruction (Young syndrome) Varicocele |
Volume abnormality |
|
No ejaculate | Ductal obstruction Retrograde ejaculation Ejaculatory failure Hypogonadism |
Low volume | Ductal obstruction Absence of seminal vesicles and vas deferens Retrograde ejaculation Infection |
Motility/morphology dysfunction |
|
Abnormal motility | Autoimmunity Infection Varicocele Sperm structural defects Metabolic abnormalities of sperm Abnormal viscosity Poor liquefaction of semen |
Abnormal morphology | Varicocele Stress Infection |
• Tubal damage, uterine anomaly. Tubal deformity or blockage, intrauterine congenital anomalies, intrauterine adhesions, and leiomyomas are uncommon causes of infertility and can be diagnosed with ultrasound or hysterosalpingogram. These conditions may require surgical correction via laparoscopy or laparotomy with tubal microsurgery, although pregnancy outcomes may be more cost effectively achieved via in vitro fertilization (IVF).7
• Endometriosis. The treatment of infertile women with endometriosis depends on the degree and location of the endometrial deposits. Conservative surgical treatment may enhance fertility by destroying endometrial implants and endometriomas. The laparoscopic cauterization of early-stage endometriosis has been shown to improve pregnancy rates. Ovulation suppression by danazol, progestins, and gonadotropin-releasing hormone analogues has been shown not to be effective in the treatment of endometriosis-associated infertility. Superovulation with clomiphene or human menopausal gonadotropins has been shown to be effective in such patients.3,7
Prognosis, Referral
The specific prognosis of infertility is difficult to determine due to the multiple etiologies. For most causes of infertility, conception will not occur without specific treatment. However, favorable pregnancy rates are reported when specific therapy is instituted. If the comprehensive diagnostic workup fails to establish a diagnosis or if appropriate treatment is unsuccessful, the physician should consider referring the couple to an infertility specialist for additional treatment and consideration of intrauterine insemination or IVF. The options for adoption should also be discussed.3
REFERENCES
1. Fritz MA, Speroff L. Clinical gynecologic endocrinology and infertility. 8th ed. Baltimore, MD: Williams & Wilkins; 2010.
2. Kaplan JL, Porter RS, eds. 2011. Merck manual of diagnosis and therapy. 19th ed. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2011.
3. Hull MG, Glazener CM, Kelly NJ, et al. Population study of causes, treatment, and outcome of infertility. Br Med J 1985;14(291):1693–1697.
4. Ghadir S, Ambartsumyan G, DeCherney AH. Infertility. In: DeCherney AH, Nathan L, Laufer N, et al., eds. CURRENT diagnosis & treatment: obstetrics & gynecology. 11th ed. New York, NY: McGraw-Hill; 2013:chap 53.
5. Mohan SK, Siladitya B. Demographics of infertility and management of unexplained infertility. Best Pract Res Clin Obstet Gynecol 2012;26(6):729–378.
6. Collins JA, Burrows EA, Willan AR, et al. The prognosis for live birth among untreated infertile couples. Fertil Steril 1995;64(1):22–28.
7. Lentz GM, Lobo RA, Gershenson DM, et al. Comprehensive gynecology. 6th ed. Philadelphia, PA: Elsevier Mosby; 2012.
8. Mishell DR, Davajan V, Lobo RA, eds. Infertility, contraception and reproductive endocrinology. 3rd ed. Cambridge, MA: Blackwell Scientific; 1991.
| Genetic Disorders and Pregnancy |
GENERAL PRINCIPLES
Genetic disorders in pregnancy is a complex topic for patients and providers. For most genetic conditions, the benefits of prenatal diagnosis have not been scientifically evaluated, other than the option for a patient to terminate a pregnancy affected by a genetic disorder. Patients may be concerned about possible discrimination against themselves or future offspring. Genetic counseling is recommended to clarify these issues and discuss the implications of testing for patients who are at risk. This chapter reviews the most common genetic disorders found during pregnancy, specifically the ones for which screening tests are available.
Types of Genetic Disorders
• Chromosome disorders are caused by the loss, gain, or abnormal arrangement of one or more chromosomes. The incidence of these disorders in the population is about 0.2%. Down syndrome (trisomy 21) is discussed below.
• Mendelian disorders are single-gene disorders caused by a mutant allele at a single genetic locus. The transmission pattern is further divided into autosomal dominant, autosomal recessive, X-linked dominant, and X-linked recessive. The incidence of these disorders is about 0.35%. Screening for cystic fibrosis, hemoglobinopathies, and Tay–Sachs disease is discussed below.
• Multifactorial disorders involve interactions between genes and environmental factors. The nature of these interactions is poorly understood. Screening for cardiac defects and neural tube defects (NTDs) is described below.
CHROMOSOME DISORDERS: DOWN SYNDROME
Down syndrome (trisomy 21) occurs in 1 in 700 live births. The risk of having a fetus with Down syndrome increases with maternal age. Affected children have variable degrees of intellectual disability (mean IQ 50). About 40% to 50% of those children will have congenital heart defects, and there is an increased risk of duodenal atresia and tracheoesophageal fistula. Affected children also have increased problems with hearing, vision, hypothyroidism, leukemia, cervical spine instability, and Alzheimer disease.
Noninvasive Prenatal Screening for Down Syndrome
Various tests can be done though serum detection or ultrasound during the first and second trimester, including combined test, quadruple test, integrated test, and serum integrated test. The American College of Obstetrics and Gynecology (ACOG) recommends that all women be offered serum screening during their pregnancy,1 Both the first and second trimester serum screening are simple to perform and have high rates of sensitivity with small false positive rates. Second trimester ultrasound is readily available but has a lower sensitivity for detection of Down syndrome.
• Early testing allows women who would choose termination of the pregnancy to do so at an earlier gestational age (when termination is safer). For women who would not choose termination, determination of the fetal abnormality allows patients to prepare for the baby and its added needs. It also allows providers to do further testing on the fetus (i.e., cardiac echo). Identification of pregnancies that have a fetus affected by Down syndrome will also assist physicians in management of the pregnancy. These pregnancies often have higher rates of pre-eclampsia, postdates pregnancy, and dysfunctional labor.
• First trimester screening. Serum and ultrasound markers that can be evaluated during weeks 10 to 13 of gestation. The serum markers are pregnancy-associated plasma protein A (PAPP-A) and the free unit of β-human chorionic gonadotropin (hCG). PAPP-A levels are 2.5 times lower in fetuses with Down syndrome and levels of the free unit of β-hCG are two times higher.
• Increased nuchal translucency (NT) seen on ultrasound during weeks 11 to 13 of gestation is associated with Down syndrome, trisomy 18, and other aneuploidies. The combined test (serum markers and ultrasound for NT, together with maternal age) is recommended as an effective screening test in the general population with an approximate sensitivity of 85% and a false-positive rate (FPR) of 5%. It also can be used to detect trisomy 18.2 However NT measurement requires specialized training and thus is not available in some regions. Women who have first trimester screening for Down syndrome still need to have an α-fetoprotein (AFP) level done in the second trimester for evaluation of NTDs.
• The cost-effectiveness of combined screening in the first trimester is debatable. Compared to second trimester screening, performing fewer amniocenteses decreases the cost, while measuring NT with its high FPR increases the cost.
• Second trimester screening. The quadruple test is a serum study that evaluates AFP, β-hCG, inhibin A, and estriol levels. The test can be performed from 15 to 22 weeks of gestation and has a sensitivity of 85% with FPR of 5% to 6%.3 Calculation of the serum marker levels combined with maternal age provides a personal risk score for each woman for her risk of having a fetus with Down syndrome. The screening test is considered positive if the calculated risk is greater than 1/270. This is the risk of a 35-year-old woman having a fetus with Down syndrome and is also the risk of a procedure-related loss from invasive testing for Down syndrome.
• Incorrect gestational age is the most common reason for a false-positive result. Gestational age should always be first confirmed with an ultrasound prior to proceeding to invasive testing for an evaluation of a positive screen.
• Ultrasound. Second trimester ultrasound can be used to identify major and minor structural abnormalities of the fetus that are associated with Down syndrome. Major structural abnormalities include heart defects and duodenal atresia. Minor ultrasound markers include increased nuchal fold, echogenic bowel, pyelectasis, echogenic cardiac focus, choroids plexus cysts, two-vessel cord, and absent nasal bone. Sensitivity for detection of Down syndrome with one or more makers is 79% with a FPR of 12%.
• Integrated test. The test integrates both first and second trimester measurements into a single test result. It typically includes NT and PAPP-A in the first trimester and quadruple test in the second trimester. Compared to combined test or quadruple test alone, it achieves an equivalent sensitivity (85%) with a much lower FPR of 1%. When measurement of NT is not available, serum integrated test can be used as an alternative (sensitivity 85% and FPR 4.4%).
Invasive Diagnostic Test
This test is available both to evaluate a positive screen for Down syndrome and also as primary screening for women over the age of 35.
• Chorionic villus sampling (CVS) can be performed from 10 to 13 weeks of gestation. A cannula is used to obtain a small amount of placental tissue via a transcervical (TC-CVS) or transabdominal (TA-CVS) approach. This provides a large amount of genetic material and karyotype results are often available in 48 hours.
• Risks of the procedure include spontaneous fetal loss. The relative risk of fetal loss compared to amniocentesis is 1.3 (CI 1.2 to 1.5). However, when rates of TC-CVS are removed, the rate of loss is similar to amniocentesis. Other risks include bleeding, infection, and fetomaternal hemorrhage.
• Amniocentesis can be performed as early as 13 weeks gestation, but is more commonly done after 15 weeks. A needle is introduced into the amniotic cavity and amniotic fluid is removed. Sloughed fetal cells are cultured and DNA is extracted for karyotyping. Results can take up to 2 weeks.
• Total fetal loss after amniocentesis is 6.1% with a procedure-related loss of 0.6%. Membrane rupture occurs 1.7% of the time, but most of the leaks are small, resolve spontaneously, and reaccumulate within a week. Other risks include indirect fetal injury, infection, and fetomaternal hemorrhage.
MENDELIAN DISORDERS
Cystic Fibrosis
Cystic fibrosis (CF) is the most common autosomal recessive disorder of Caucasians of Northern European descent. The carrier rate for a mutation is 1 in 25. Carrier rates are also high in Ashkenazi Jews (1 in 24). CF can cause abnormal pulmonary function, pancreatic insufficiency, or congenital absence of the vas deferens. The average age of survival is about 37 years and continues to increase.
• Serum screening can be done at any time, but ideally is best done prior to pregnancy. Because most screening tests detect only 90% of the mutations that cause CF, screening can decrease risk but not eliminate it.4
• The ACOG recommends that preconception and prenatal CF carrier screening be offered to all women of reproductive age.5 Women who are not in high-risk groups (family history of CF, reproductive partner with CF, Caucasian of European descent, or Ashkenazi Jews) should be offered the test, but the decreased detectability of mutations should be discussed. If the patient is a carrier, screening should be offered to her partner. It is recommended to offer genetic counseling to couples in which both partners are carriers.
• The fetus can be tested for CF with amniocentesis, CVS, or fetal blood sample. Issues that need to be considered include the related fetal loss associated with invasive testing. Knowledge of the diagnosis for the fetus will most likely not change the neonatal course (except for monitoring/treatment of meconium ileus). In addition, parents need to be reminded that the same genotypes often have different phenotypic presentations, which makes prediction of morbidity and mortality from CF problematic.
Hemoglobinopathies
Hemoglobinopathies have an autosomal recessive inheritance pattern. High-risk groups are people of African, Southeast Asian, and Mediterranean descent. Complete blood count (CBC) is an appropriate first-line screening test for most at-risk women. Additionally, ACOG recommends that all women of African descent have hemoglobin electrophoresis at the same time.6
Women of Southeast Asian or Mediterranean descent with a low mean corpuscular volume (MCV) on CBC testing (less than 80 μ3) should have hemoglobin electrophoresis to determine whether they are hemoglobinopathy carriers.
Prenatal genetic testing by CVS or amniocentesis is available for sickle cell disease, and for α- and β-thalassemia when the mutations have previously been identified in the parents.
Sickle Cell Disease
Sickle cell disease is the most common hemoglobinopathy; 1 out of 12 African Americans has sickle cell trait. Sickle cell trait can be detected by hemoglobin electrophoresis. If a woman who is pregnant or contemplating pregnancy tests positive, the next step is testing of the male partner. If both partners test positive, the couple should be referred for genetic counseling. Despite the relatively high prevalence of sickle cell disease, surveys have shown that most African American women of childbearing age do not understand the inheritance pattern or implications of the carrier state.
Thalassemia
α-Thalassemia trait (α-thalassemia minor) has two variants. In individuals of Southeast Asian descent, both α-globin genes on the same chromosome are deleted. They are at higher risk for a child with hemoglobin H disease (deletion of three of the four α-globin genes) and hemoglobin Bart (or α-thalassemia major, deletion of all four α-globin genes). Hemoglobin H results in mild to moderate hemolytic anemia. Hemoglobin Bart can lead to hydrops fetalis, intrauterine fetal demise, and pre-eclampsia.
In individuals of African descent with α-thalassemia trait, one α-globin gene is deleted on each copy of chromosome 16. They are not typically at risk for offspring with hemoglobin H or hemoglobin Bart. However, mild anemia is often present.
α-Thalassemia trait will not be detected on hemoglobin electrophoresis. DNA-based testing is needed for women with low MCV, no iron-deficiency anemia, and a normal hemoglobin electrophoresis.
Tay–Sachs Disease
The carrier rate for Tay–Sachs disease is 1 of 30 for people of Eastern European Jewish descent (Ashkenazi); people of French–Canadian and Cajun descent also have higher rates. Inheritance is autosomal recessive.
In this disorder, accumulation of gangliosides in the central nervous system results in progressive neurologic disease and death in early childhood.
In nonpregnant women, carrier screening can be performed by molecular or biochemical analysis.7 Biochemical analysis has a higher carrier detection rate, especially in low-risk populations. However, in women who are pregnant or taking oral contraceptives, serum biochemical testing may yield a false-positive result. These women should have biochemical testing on peripheral leukocytes or molecular testing. Genetic counseling is recommended for high-risk patients to determine the correct type of testing and to interpret the test results.
MULTIFACTORIAL DISORDERS
Congenital Heart Disease
Congenital heart disease (CHD) is the most common congenital anomaly, occurring in 8 of 1,000 live births. Risk factors include family history of CHD, maternal diabetes, exposure to cardiac teratogens, noncardiac fetal anomalies detected on ultrasound, chromosomal abnormalities, single umbilical artery, fetal arrhythmias, nonimmune fetal hydrops, and increased NT.
The majority of CHD will occur in a low-risk population. In the United States, although routine ultrasound screening is not mandated, most women do undergo ultrasound screening during pregnancy. The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends routine cardiac screening should include both four-chamber view and outflow tract views. The detection rate of CHD on routine 18- to 22-week ultrasound is very variable, ranging from 23% to 77%.8 However, women with risk factors should be referred for fetal echocardiography.9
The advantages of early detection of CHD fall into three categories: offering patients the choice of pregnancy termination, prenatal intervention, and postnatal management. Prenatal intervention is still in the experimental stages. Although postnatal management, such as delivery at a tertiary care center and preparation for early surgery, would appear to have logical benefits, the advantages of this have been difficult to demonstrate in studies.
Neural Tube Defects
NTDs are the second most prevalent congenital anomaly. In the United States, NTDs occur in 1 in 1,000 pregnancies. The defects either appear in the spine (spina bifida, meningomyelocele, meningocele) or the cranium (anencephaly, which accounts for 50% of NTDs). Some NTDs are associated with genetic syndromes, but the majority are isolated defects.
NTDs most likely result from a combination of genetic and environmental influences. In a family with a child with a previous NTD, the relative risk of having another child with an NTD is 2% to 4%. Exposure to drugs that interfere with folic acid metabolism (carbamazepine, valproic acid) can cause NTDs. Maternal hyperthermia, diabetes, and obesity also increase the risk of having a fetus with an NTD. Finally, folic acid deficiency is known to increase the risk of NTDs. Preconception and early gestation (prior to 6 weeks) supplementation decrease the risk of NTDs.
Screening
Since 90% of NTDs occur in women with no prior history of having an affected fetus, it is recommended that all pregnant women be offered screening during weeks 15 to 20 of gestation.10
• Maternal serum α-fetoprotein (MSAFP) is elevated in 89% to 100% of pregnancies with a fetal NTD. An MSAFP greater than 2.0 to 2.5 multiples of the mean is 75% to 90% sensitive (5% FPR) for detection of all NTDs and greater than 95% sensitive (2% to 5% FPR) for detection of anencephaly.11 Some factors such as gestational age, maternal weight, diabetes mellitus, fetal abnormality, and multiple gestations will affect the test results. Follow-up ultrasound examination should be offered to patients with positive MSAFP results.
• Ultrasound is used as both a screening and diagnostic examination, especially in high-risk pregnancies. It is 97% to 100% sensitive for the detection of NTDs and should be offered to all women with an abnormal MSAFP or women who are at high risk for a fetus with NTD during 18 to 20 weeks of gestation.
• Amniocentesis allows for removal of amniotic fluid and evaluation of amniotic fluid AFP (AFAFP) and acetylcholinesterase. Amniocentesis should be considered if the ultrasound is equivocal or normal with positive MSAFP result, or if the patient wishes to have karyotype determination of the fetus. If both AFAFP and amniotic acetylcholinesterase levels are abnormal, the fetus most likely has an open NTD (96% sensitivity, 0.14% false positive).
• Fetal magnetic resonance imaging (MRI) can be considered in cases where ultrasound failed to obtain ideal visualization. However, the diagnostic capability and efficacy needs further investigation.
OTHER CONGENITAL ANOMALIES
The rates of ultrasound detection of anomalies vary from over 90% for major defects such as hydrocephalus and anencephaly, to just over 17% for cleft lip and palate and foot deformities.12 Because of this wide variability, the value of ultrasound screening for congenital anomalies is still unproven. Pregnant women should be informed about the detection rates of anomalies on ultrasound, and that many anomalies may be missed on ultrasound testing.
REFERENCES
1. ACOG Committee on Practice Bulletins. ACOG practice bulletin No. 77: screening for fetal chromosomal abnormalities. Obstet Gynecol 2007;109(1):217–227.
2. Messerlian GM, Farina A, Palomaki GE. First trimester combined test and integrated tests for screening for down syndrome and trisomy 18. www.uptodate.com. Accessed January 15, 2014.
3. Messerlian GM, Farina A, Palomaki GE. Second trimester maternal serum screening for Down syndrome. www.uptodate.com. Accessed January 15, 2014.
4. Wenstrom KD. Cystic fibrosis: prenatal genetic screening. www.uptodate.com. Accessed on December 30, 2013.
5. American College of Obstetricians and Gynecologists Committee on Genetics. ACOG committee opinion No. 486: update on carrier screening for cystic fibrosis. Obstet Gynecol 2011;117(4);1028–1031.
6. ACOG Committee on Obstetrics. ACOG practice bulletin No. 78: hemoglobinopathies in pregnancy. Obstet Gynecol 2007;109(1):229–237.
7. ACOG Committee on Genetics. ACOG committee opinion. Number 318, October 2005. Screening for Tay–Sachs disease. Obstet Gynecol 2005;106(4):893–894.
8. Copel J. Prenatal sonographic diagnosis of fetal cardiac anomalies. www.uptodate.com. Accessed January 15, 2014.
9. Carvalho JS, Allan LD, Chaoui R, et al.; International Society of Ultrasound in Obstetrics and Gynecology. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol 2013;41:348–359.
10. Driscoll DA, Gross SJ; Professional Practice Guidelines Committee. Screening for fetal aneuploidy and neural tube defect. Genet Med 2009;11(11):818–821.
11. Hochberg L, Stone J. Prenatal screening and diagnosis of neural tube defects. www.uptodate.com. Accessed January 15, 2014.
12. American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 101: ultrasonography in pregnancy. Obstet Gynecol 2009;113(2):451–461.
|
GENERAL PRINCIPLES
Family-centered prenatal care is the delivery of effective, efficient, accessible, safe, and economical quality care for the psychosocial, spiritual, and physical needs of the mother, child, father, and family unit. Family physicians with this philosophy view childbirth as a vital life event in the family and a foundational event in the formation of community and society. The family physician is ideally suited to provide this care, whether the family physician will deliver the baby or provide “shared prenatal care” with a delivering midwife or physician. An in-depth review of routine prenatal care is beyond the scope of this chapter; however, basic information that is frequently needed during prenatal care is provided, and Figure 14.4-1 illustrates important considerations and decision points in prenatal care.
DIAGNOSIS
History
A comprehensive history is required to provide appropriate prenatal care and distinguish between uncomplicated and higher risk obstetrical patients. Ideally, prenatal care begins before conception and includes preventive, health-maintenance care, counseling, and screening for risks to maternal and fetal health.1 The following components should be included:
• Medical/surgical history. History of diabetes, hypertension, asthma, seizure disorder, mental illness, hematologic disorders, cancer, HIV, recurrent urinary tract infections. Past surgeries. Previous history of varicella.
• Maternal care history. Past history of complicated pregnancy or delivery. History of preterm delivery; prior cervical or uterine surgery. History of fetal anatomic abnormality or intrauterine fetal demise.
• Psychosocial history. History of eating disorder, substance use, mood disorder, psychosis, or postpartum depression.
• Family history of medical and genetic disorders; prescription and over-the-counter medication use; substance use; history of domestic violence; history of transfusions; and immunization status. Ascertain risk for tuberculosis as well as sexually transmitted infections (STIs) or diseases (STDs) caused by HPV, HIV, hepatitis B or C, gonorrhea, Chlamydia, herpes, or syphilis.1
Physical Examination
• Initial visit. The estimated date of delivery (EDD) is ascertained based on the patient’s last menstrual period (LMP). Early ultrasound is indicated to determine the EDD whether there is uncertainty about the LMP.
• Examination should include height, weight, and body mass index (BMI); blood pressure; screening Pap smear for women who have not been recently screened and meet criteria. Obtain cervical cultures for gonorrhea (GC) and Chlamydia (Chl) in high-risk women (age <25 years; unmarried; Black, a history of STIs or STDs, new or multiple sexual partners, inconsistent use of barrier contraception; and living in communities with high infection rates).1
• Initial lab includes blood type and Rh, HIV, rubella, hepatitis B surface antigen (HBsAg), urine culture; varicella titer if unsure of history; and urine dipstick to determine baseline renal function.1
• All subsequent visits should include interval history and address any patient concerns or symptoms suggestive of preeclampsia or preterm labor, along with assessment of blood pressure, weight gain, fundal height, and fetal heart tones.
• Beginning with the start of the third trimester, visits should also include an assessment of fetal movement and abdominal palpation to assess fetal presentation at 36 weeks gestation.1
• There is no benefit in performing routine urine dipstick to check for proteinuria or glycosuria in low-risk women.2
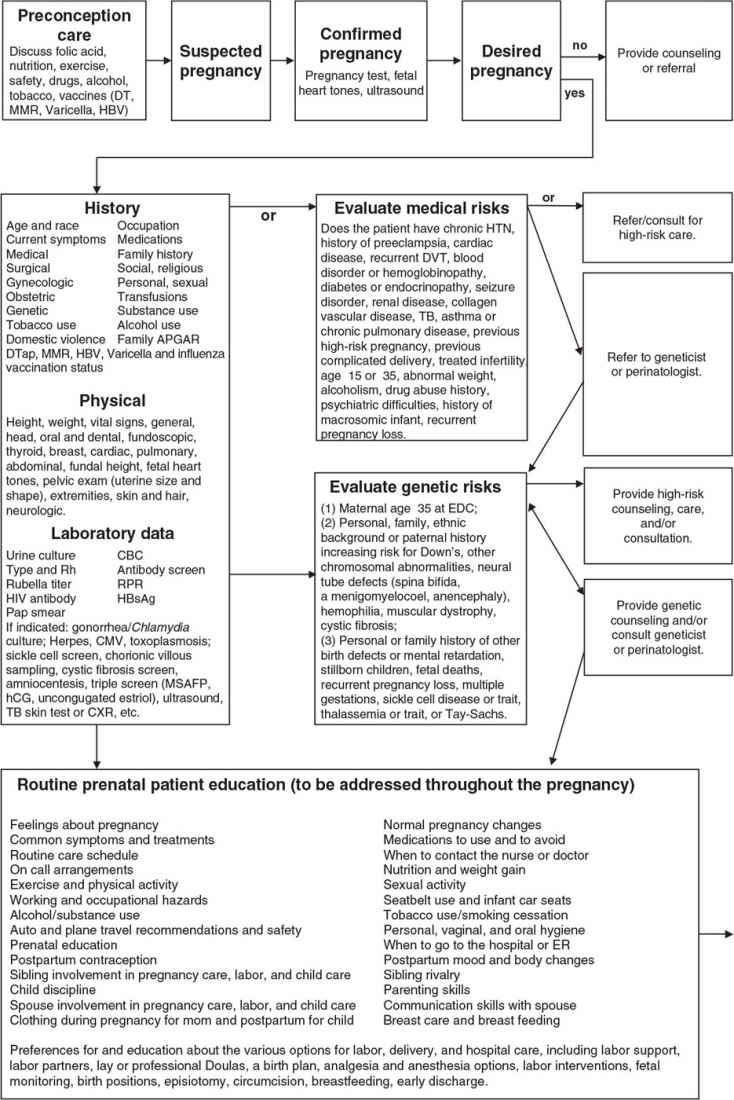
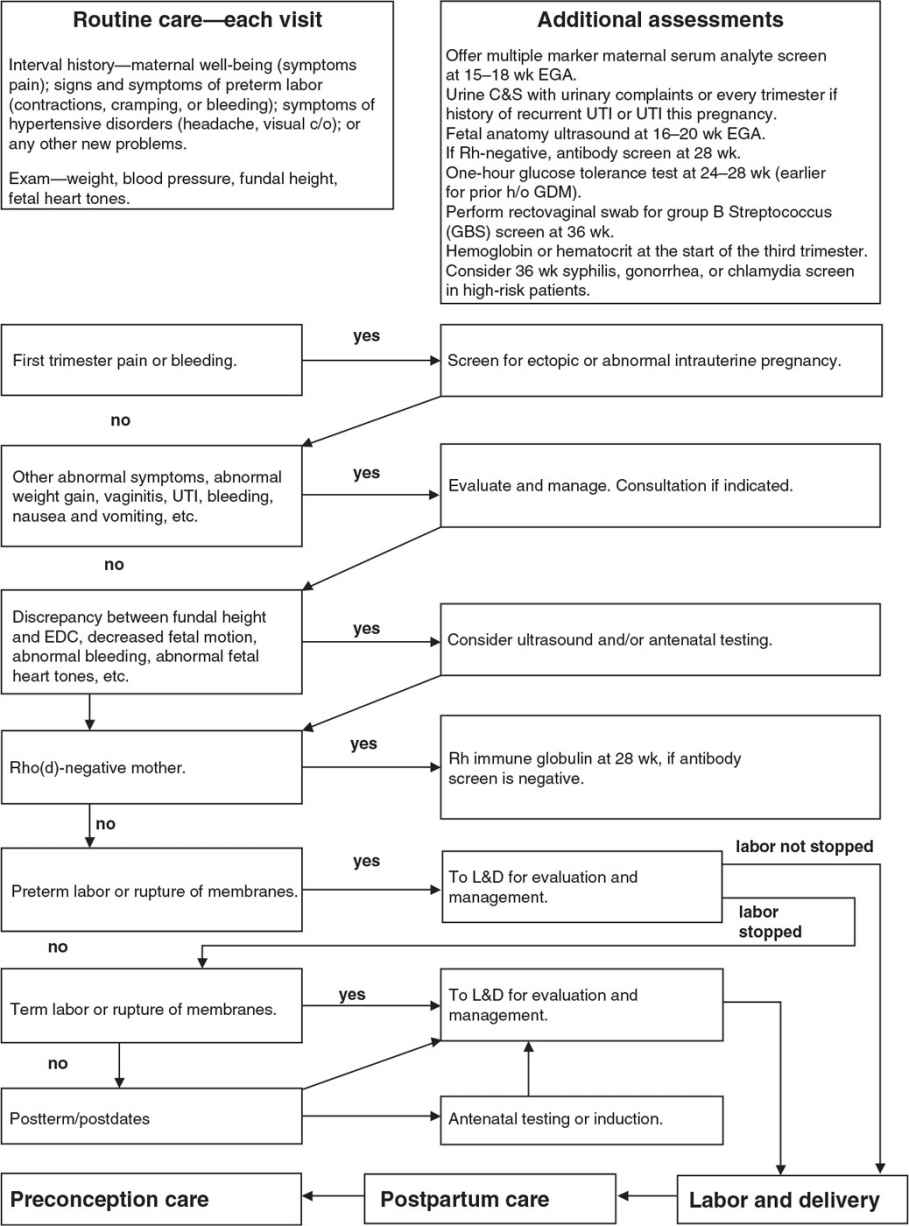
Figure 14.4-1. Important considerations and decision points in prenatal care. TB, tuberculosis; UTI, urinary tract infection; C&S, culture and sensitivity; CBC, complete blood count; CMV, cytomegalovirus; c/o, complains of; CXR, chest x-ray; DTap, diphtheria, tetanus, acellular pertussis vaccine; DVT, deep venous thrombosis; EDC, estimated date of confinement (due date); EGA, estimated gestational age; ER, emergency room; GDM, gestational diabetes mellitus; HBsAg, hepatitis B surface antigen; HBV, hepatitis B vaccine; hCG, human chorionic gonadotropin; HIV, human immunodeficiency virus; h/o, history of; HTN, hypertension; L&D, labor and delivery; MMR 5, measles, mumps, rubella vaccine; MSAFP, maternal serum α-fetoprotein; Rh, Rhesus factor; RPR, rapid plasma regain.
TREATMENT, INTERVENTIONS/RECOMMENDATIONS
Behavioral Counseling
• Nutrition and weight gain. Most pregnant women require an increase of 300 to 400 calories per day above nonpregnant levels throughout pregnancy.3 For most patients, a well-balanced diet provides adequate nutrition during pregnancy. In general, special diets, skipping meals, and food avoidance may lead to nutritional deficiencies and inadequate weight gain during pregnancy. Recommendations for weight gain are based on a pre-pregnancy ideal body weight (IBW) or BMI.3 The average weight gain in women with a normal BMI (18.5 to 24.9) is 25 to 35 pounds. Women who enter pregnancy substantially below their IBW (BMI less than 18.5) should gain a greater amount of weight during pregnancy (e.g., 28 to 40 pounds). Overweight (BMI 25 to 29.9) women should gain 15 to 25 pounds, and obese (BMI >30) women should be advised to gain less weight during pregnancy 11 to 20 pounds during pregnancy.4
• Iron is necessary to expand maternal red cell mass and for fetal–placental development. Iron consumption should be increased to 27 mg per day, the amount found in most prenatal vitamins. Dietary sources include red meat, poultry, fish, whole grains, dried fruits, green leafy vegetables, and legumes, seeds, and nuts. Vitamin C enhances iron absorption from plant foods when taken with a meal. Women with iron deficiency anemia should receive an additional iron supplement of 30 to 120 mg per day until the anemia is corrected.5 There is no evidence for routine as opposed to selective iron supplementation in populations with a low prevalence of iron deficiency.
• Folate supplementation is recommended to reduce the risk of neural tube defects (NTDs). Supplemental folate is ideally recommended in the pre-conceptual period, as the neural tube closes between 18 and 26 days after conception. The Centers for Disease Control and Prevention (CDC) recommend all low-risk fertile women to take 400 μg of folic acid daily, and increase to 600 μg daily during and throughout pregnancy.5 Women at increased risk for offspring with NTDs should take higher pre-pregnancy doses (4 mg per day). This includes personal or family history of NTD, maternal insulin-dependent diabetes, and women who are taking anticonvulsants.5
• Calcium is required for fetal skeletal development, particularly in the last trimester. Calcium absorption is increased during pregnancy and, if necessary, is easily mobilized from maternal stores. During pregnancy and lactation, elemental calcium intake should include at least 1,000 mg per day in women 19 to 50 years old; for women and girls 14 to 18 years old, 1,300 mg of calcium is recommended daily.5
• Proteins are a critical part in the fetus’s proper brain development. As such, pregnant women are advised to ingest an additional 5 to 6 g of protein daily above the nonpregnant state.3
• Vegetarian diets may not provide adequate amounts of essential amino acids, iron, vitamin B12, or complex lipids for normal embryonic development. Minor dietary alterations, such as increasing soy, legumes, and dairy products, may correct these deficiencies. Consultation with a registered dietician for further recommendations is advised.5
• Mega-vitamins and natural medications (herbs, vitamins, and supplements). Inquire about consumption of these substances. Excessive intake of these substances may prove toxic and possibly teratogenic.
• Foods to limit or avoid due to potentially adverse effects: high caffeine intake (more than 200 mg daily), unwashed produce, unpasteurized dairy products, undercooked meats, and fish potentially containing high levels of mercury.5
• Exercise. At least 30 minutes of moderate exercise on most days of the week is reasonable for most pregnant women. Pregnant women should avoid activities that put them at risk for falls or abdominal injuries.
• Immunizations. Live-virus vaccines are generally contraindicated for pregnant women because of the theoretical risk of transmission of the vaccine virus to the fetus. If a live-virus vaccine is inadvertently given to a pregnant woman, or if a woman becomes pregnant within 4 weeks after vaccination, she should be counseled about the potential effects on the fetus; however, it is not typically an indication to terminate the pregnancy.6
• Inactivated influenza vaccine is recommended for all women who are or will be pregnant during flu season.6
• Tetanus/diphtheria/pertussis (Tdap) is recommended during each pregnancy, regardless of the last time vaccination occurred. Vaccination may be given at any time during pregnancy, but administration between 27 and 36 weeks provides optimal timing for antibody transfer to the fetus to occur.7
• Live vaccines are contraindicated during pregnancy: influenza (live-attenuated), measles, mumps, rubella, varicella.
• The following vaccines can be safely administered if clinically indicated: Hepatitis A, Hepatitis B.
• Recommendations for travel and other circumstances are published at the CDC Web site.6
• Medications. Few medications have been proven to be completely safe for use in pregnant women, especially in the first trimester. Benefits and risks must be carefully weighed before initiating prescription, over-the-counter, or natural medications.1
Patient Education
• Breastfeeding education. Breastfeeding education should be offered to all pregnant women at their first visit with the provider and should be encouraged throughout the pregnancy.1
• Preterm labor precautions. Pregnant women should be educated about the most common symptoms of preterm labor: low, dull backache; four or more uterine contractions per hour; increased pelvic pressure; change in vaginal discharge.1
• Labor and delivery. Pregnant women should be counseled about signs of labor, ruptured membranes, pain management, and what to expect in labor.1
• Injury prevention. Seat belts should be properly worn.1
Screening
• Substance abuse. All pregnant women should be screened for tobacco, alcohol, and illicit substance use.1
• Domestic violence. Domestic violence affects a significant number of pregnant women and may put both the woman and her fetus at risk. Patients generally accept screening questions about domestic violence.8
• Asymptomatic bacteriuria. Routine urine culture is recommended due to increased risk of pyelonephritis among pregnant women with asymptomatic bacteriuria.9 Perform screening urine culture and sensitivity (C&S) at the initial visit. For patients with history of recurrent urinary tract infections (UTIs), UTI during pregnancy, or symptomatic infections, urine culture should be repeated each trimester.9
Genetic Screening
• Chorionic villi sampling may be offered between 10 and 12 weeks estimated gestational age (EGA). It is associated with 1% to 1.5% risk of spontaneous abortion (SAB) and may be associated with transverse limb defects. Amniocentesis may be offered after 15 weeks EGA and is associated with a 0.5% risk of SAB.10
• Maternal serum analyte screen should be offered at 15 to 20 weeks EGA to screen for NTDs and trisomies 21 and 18. Optimal timing is 15 to 18 weeks EGA to maximize accuracy and allow time for adequate follow-up counseling and testing.11
• Fetal anatomy ultrasound can be offered at 16 to 20 weeks EGA to evaluate for structural anomalies.12
• Antibody screen for Rh-negative women at the first prenatal visit, and again at 28 weeks EGA.
• Administer Rho(D) immune globulin antepartum at 28 weeks and postpartum (if the baby is Rh-positive) to prevent hemolytic disease of the newborn in subsequent pregnancies.13
• Rho(D) immune globulin is also indicated for spontaneous or induced abortion, ectopic pregnancy, chorionic villus sampling, amniocentesis, vaginal bleeding, significant abdominal trauma, external cephalic version, and transfusion of unmatched Rh-positive blood or any platelet transfusion.13 For a more detailed discussion, see Chapter 14.8.
• Gestational diabetes mellitus (GDM) screening at 24 to 28 weeks (earlier if at increased risk).
• Risk factors include obesity, history of miscarriage or fetal death, age 40 or older, history of premature infant, family history of diabetes, polyhydramnios, history of infant with macrosomia (>4,000 g) or congenital malformation, pre-eclampsia, excessive weight gain, and glycosuria.14
• Screening is based on risk factors. The American Diabetes Association recommends that all pregnant women be screened with a 50-g nonfasting glucose challenge at 24 to 28 weeks. Women at high risk of GDM should be screened at the first antepartum visit using the same 50-g nonfasting glucose challenge.14 Women with a plasma glucose exceeding 140 mg per dL during a 1-hour glucose tolerance test (GTT) need a 3-hour GTT. Because of poor specificity, the use of random or fasting glucose values is not recommended as a screening tool for GDM.14
• Diagnosis. Administer a 3-hour fasting GTT with a 100-g glucose load. Two or more of these plasma values (not fingerstick values) must be met or exceeded for diagnosis of GDM: fasting, 95 mg per dL; 1 hour, 180 mg per dL; 2 hours, 155 mg per dL; and 3 hours, 140 mg per dL.14
• Group B Streptococcus (GBS) screening. Perform a rectovaginal swab at 35 to 37 weeks EGA. Colonized women, women with GBS bacteriuria, and women with a previous child with early-onset GBS infection should be treated with intravenous antibiotics at the time of labor or ruptured membranes.15
• Screening for STIs. Consider syphilis, gonorrhea, or Chlamydia screening in the third trimester in high-risk patients.
• Bacterial vaginosis. The United States Preventative Services Task Force (USPSTF) recommends against screening for bacterial vaginosis in asymptomatic women at low risk for preterm delivery. In women with a history of a previous preterm birth, there is insufficient evidence to assess whether the benefits outweigh the harms of screening asymptomatic women.16
SPECIAL CONSIDERATIONS
• Nausea and vomiting of pregnancy (NVP), also called morning sickness, is experienced by more than 70% of pregnant women.
• Lifestyle modifications should encourage small frequent protein meals and increased rest. Avoidance of fried and heavily seasoned food, noxious odors, and environmental stimuli should also be recommended.
• Nonpharmacologic treatments include biofeedback and self-hypnosis. Ginger suppresses gastric contractions and increases gastrointestinal motility. Ginger (250 mg oral capsules taken four times daily) significantly reduced nausea and vomiting compared with placebo in women who were <17 weeks pregnant.17
• Oral pharmacologic treatments
• Pyridoxine (vitamin B6) is considered first-line treatment for NVP: 12.5 to 25 mg TID, or pyridoxine, 25 to 50 mg PO TID–QID used in combination with doxylamine, 10 to 12.5 mg PO QD–BID.18 Doxylamine is available over the counter in 12.5-mg (Decapryn) and 25-mg tablets (Unisom Nighttime Sleep-Aid Tablets). The latter combination is contained in the prescription drug Diclegis.19
• Antiemetics, antihistamines, phenothiazines, and benzamides are common prescription oral medications for NVP. An algorithm for the suggested evaluation and treatment with NVP is available.18
• Newer data indicate that hyperemesis gravidarum may be associated with Helicobacter pylori infection. H. pylori infection may be safely and effectively treated with triple therapy (amoxicillin, metronidazole, and an H2-receptor blocker) after the first trimester of pregnancy.20
• Vaginal bleeding is common in early pregnancy, occurring in approximately 20% to 40% of all pregnant patients. Any report of bleeding deserves further investigation to delineate between potentially serious and less worrisome causes.21
• Evaluation should include a physical examination, laboratory studies (quantitative β-hCG and progesterone, blood type and Rh, serology, and cultures when indicated), and ultrasound. First trimester etiologies include threatened or spontaneous abortion, ectopic pregnancy, trophoblastic disease, cervical polyps, friable cervix, trauma, or malignancy. Bleeding that occurs in the second and third trimesters warrants immediate investigation for abnormally implanted placenta, placental abruption, or other potentially serious conditions21 (see Chapter 14.10).
• Vaginal discharge is common in pregnancy, with many women noting increased vaginal discharge during pregnancy. If this becomes symptomatic, further investigation is warranted using culture and microscopic techniques. Optimal treatment for symptomatic vulvovaginal candidiasis consists of a topical imidazole (clomitrazole or miconazole) for 7 days.22
• Treatment of symptomatic bacterial vaginosis may be achieved with a topical or oral agent. Whether treatment of bacterial vaginosis with oral metronidazole reduces the risk of preterm delivery remains unclear.22 Increased vaginal discharge or secretions in the second or third trimester may signal preterm cervical changes or labor and may warrant sterile vaginal examination.
• Back pain and pelvic pain is common in pregnancy and aggravated by mechanical and hormonal factors. It occurs in more than two-thirds of pregnancies, and often interferes with daily activities and sleep.23 Usual recommendations include stretching and strengthening exercise, oral or topical analgesics, massage, heat therapy, or ice therapy. Acupuncture has been demonstrated to be more effective in reducing pain than physiotherapy.24
• Evidence for the use of specially shaped pillows instead of regular pillows to reduce nighttime back pain has been shown to be very low.23
• Osteopathic manipulation therapy (OMT) conducted during the third trimester of pregnancy, when combined with conventional obstetric care, has been shown to decrease back pain and slow the progression of problems involving back function.25 OMT intervention in the first and second trimesters has not been adequately studied.
• Leg cramps affect almost half of all pregnant women, particularly in the second and third trimesters of pregnancy; they mostly occur at night. Their cause is unknown, but may be associated with increased levels of lactic and pyruvic acids.26 Stretching, taking a hot shower, and walking may alleviate leg cramps. Regular exercise and increased hydration may also help.27 Evidence supporting the use of magnesium prophylaxis of pregnancy-associated leg cramps is conflicting and unclear at this time.27
• Heartburn and gastroesophageal reflux disease (GERD) may occur in the second to third trimesters and can be a source of significant discomfort.
• Nonpharmacologic interventions include eating small, frequent meals; avoiding fried, greasy, and spicy foods; and eating slowly and chewing food well. Like nonpregnant patients with these symptoms, it is helpful to avoid lying down immediately after eating, to take walks after meals, and to drink fluids between meals.
• Pharmacologic agents used to treat heartburn include:
• Antacids (calcium carbonate, magnesium hydroxide and oxide, and aluminum hydroxide and carbonate). Systemic absorption of antacids is negligible; recommended doses are safe in pregnancy and lactation.28 Antacids containing sodium bicarbonate or magnesium trisilicate should be avoided.
• Antisecretory agents used for heartburn and GERD include the histamine H2-antagonists and proton pump inhibitors. The histamine antagonists, cimetidine (Tagamet) and ranitidine (Zantac), are the most well studied; they have generally shown significant symptom improvement with minimal side effects. Nizatidine (Axid) should be avoided due to the potential risk for fetal death and spontaneous abortion, as seen in animal studies.28 Because of a lack of evidence regarding safety in the first trimester, histamine antagonists are not recommended during the first trimester. Of the proton pump inhibitors—lansoprazole (Prevacid), omeprazole (Prilosec), and pantoprazole (Protonix)—these have been most widely studied in pregnancy.29 Omeprazole and lansoprazole are available without a prescription.
REFERENCES
1. American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Guidelines for perinatal care. 7th ed. Elk Grove Village, Illinois: AAP; 2012.
2. Alto WA. No need for glycosuria/proteinuria screen in pregnant women. J Fam Pract 2005;54:978–983.
3. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician 2014;89(3):199–208.
4. American College of Obstetricians and Gynecologists. Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol 2013;121:210–212.
5. American College of Obstetrics and Gynecology Website. Nutrition during pregnancy educational pamphlet FAQ001. September 2013. http://www.acog.org/-/media/For-Patients/faq001.pdf?dmc=1&ts=20150108T1656382578
6. Guidelines for Vaccinating Pregnant Women. Abstracted from recommendations of the Advisory Committee on Immunization Practices (ACIP). April 2013. http://www.cdc.gov/vaccines/pubs/downloads/b_preg_guide.pdf
7. Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013;62(7):131–135.
8. U.S. Preventive Services Task Force. Screening for intimate partner violence and abuse of elderly and vulnerable adults. January 2013. http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed January 9, 2014.
9. Schnarr J, Smaill F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur J Clin Invest 2008;38(Suppl 2):50–57.
10. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 88, December 2007. Invasive prenatal testing for aneuploidy. Obstet Gynecol 2007;110:1459–1467.
11. American College of Obstetricians and Gynecologists Committee on Genetics. Committee opinion no. 545: noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol 2012;120:1532–1534.
12. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 101: ultrasonography in pregnancy. Obstet Gynecol 2009;113:451.
13. Crowther CA, Middleton P, McBain RD. Anti-D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev 2013;2:CD000020.
14. American College of Obstetricians and Gynecologists. Practice bulletin no. 137: gestational diabetes mellitus. Obstet Gynecol 2013;122:406–416.
15. Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–32.
16. U.S. Preventive Services Task Force. Screening for Bacterial Vaginosis in Pregnancy to Prevent Preterm Delivery, Topic Page. February 2008. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbvag.htm
17. Ozgoli G, Goli M, Simbar M. Effects of ginger capsules on pregnancy, nausea, and vomiting. J Altern Complement Med 2009;15(3):243–246.
18. American College of Obstetrics and Gynecology. ACOG (American College of Obstetricians and Gynecologists) Practice bulletin: nausea and vomiting of pregnancy. Obstet Gynecol 2004;103(4):803–814.
19. Lexi-Comp Online. Doxylamine and pyridoxine drug information. Hudson, OH: Lexi-Comp Inc. https://online.lexi.com/crlsql/servlet/crlonline. Accessed March 9, 2014.
20. Mansour GM, Nashaat EH. Role of helicobacter pylori in the pathogenesis of hyperemesis gravidarum. Arch Gynecol Obstet 2011;284(4):843–847.
21. Norwitz ER, Park JS. Overview of the etiology and evaluation of vaginal bleeding in pregnant women. In: Barss VA, ed. UpToDate. Waltham, MA: UpToDate. Accessed March 8, 2014.
22. American College of Obstetricians and Gynecologists. ACOG practice bulletin clinical management guidelines for obstetrician-gynecologists, number 72, May 2006: vaginitis. Obstet Gynecol 2006;107:1195–1206.
23. Pennick V, Liddle SD. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev 2013;8:CD001139.
24. Ee CC, Manheimer E, Pirotta MV, et al. Acupuncture for pelvic and back pain in pregnancy: a systematic review. Am J Obstet Gynecol 2008;198:254.
25. Licciardone JC, Buchanan S, Hensel KL, et al. Osteopathic manipulative treatment of back pain and related symptoms during pregnancy: a randomized controlled trial. Am J Obstet Gynecol 2010;202:43.e1.
26. Young GL, Jewell D. Interventions for leg cramps in pregnancy. Cochrane Database Syst Rev 2002;(1):CD000121.
27. Bermas BL. Musculoskeletal changes and pain during pregnancy and postpartum. In: Barss VA, ed. UpToDate. Waltham, MA: UpToDate. Accessed March 8, 2014.
28. Law R, Maltepe C, Bozzo P, et al. Treatment of heartburn and acid reflux associated with nausea and vomiting during pregnancy. Can Fam Physician 2010;56(2):143–144.
29. Gill SK, O’Brien L, Einarson TR, et al. The safety of proton pump inhibitors (PPIs) in pregnancy: a meta-analysis. Am J Gastroenterol 2009;104:1541.
|
GENERAL PRINCIPLES
Ectopic pregnancy presents a major health problem to women of childbearing age. This condition is considered a medical emergency, and hemorrhage from ruptured ectopic pregnancy is still the leading cause of pregnancy-related maternal death in the first trimester.1 Prompt diagnosis and treatment is a must in all patients presenting with signs or symptoms of ectopic pregnancy.
Definition
Ectopic pregnancy occurs when a fertilized ovum implants anywhere outside the endometrial lining of the uterine cavity. The most common location is the fallopian tube, which accounts for 97% of all ectopic gestations.2 Other, less common, sites of implantation include the abdomen, peritoneum, cervix, ovary, and uterine cornua.
Etiology
Risk factors for ectopic pregnancy can be classified into high, intermediate, and low. High degrees of risk of ectopic pregnancy occur in patients with previous ectopic pregnancy, previous tubal surgery, known tubal pathology, and in utero diethylstilbestrol (DES) exposure. Intermediate risk is attributed to patients who are smokers and have a history of pelvic inflammatory disease (PID). Low risk is seen in patients who have had previous (nontubal) abdominal surgery or in patients who become pregnant at a young age (<18).3,4 Women who are currently using an intrauterine device for contraception or who have had tubal ligation performed are much more likely to have an ectopic pregnancy if conception occurs. However, the risk of conception is obviously much lower with these contraceptive methods in place. All the aforementioned risk factors increase the chance of tubal pathology and thus tubal pregnancy.
Epidemiology
Ectopic pregnancy occurs in nearly 2 per 100 pregnancies. The incidence has increased sixfold between 1970 and 1992,2 largely due to a rise in PID. The prevalence is reported to be as high as 18% of women presenting to the emergency department with first trimester bleeding and abdominal pain.5
DIAGNOSIS
History
Pelvic/abdominal pain, amenorrhea, and vaginal bleeding are the classic symptoms of ectopic pregnancy. Pain is almost always present before rupture, but is highly variable in location, character, and severity. The onset of pain is usually at 6 to 8 weeks gestational age. Often, there is amenorrhea followed by irregular vaginal bleeding. More than 50% may be asymptomatic before tubal rupture and have no identifiable risk factor for ectopic pregnancy, making the diagnosis difficult.4
Physical Examination
Signs may include localized lower quadrant tenderness with or without a palpable mass, peritoneal irritation, guarding and rebound tenderness (suggesting tubal rupture with hemoperitoneum), and cervical motion tenderness. Signs of shock, including pallor, diaphoresis, weakness, and orthostatic pulse and blood pressure changes, may be present.4
Laboratory Tests
An initial workup should include a complete blood count (CBC), quantitative β-human chorionic gonadotropin (β-hCG), and Rh determination. The β-hCG concentration in a normal intrauterine pregnancy (IUP) rises until 41 days of gestation at which time it plateaus at approximately 100,000 IU per L and the mean doubling time for the hormone is from 1.4 to 2.1 days. In ectopic pregnancy, the doubling time is 3 or more days. A falling value signals non-viability. A single value is not interpretable and serial testing should only be used if the patient remains hemodynamically stable.5
Imaging
Transvaginal ultrasound should be the initial diagnostic test in women known to be pregnant who present with first trimester vaginal bleeding and/or pelvic pain. If the imaging study is non-diagnostic, transvaginal ultrasound findings in conjunction with serial serum β-hCG concentrations facilitate a diagnosis of ectopic pregnancy. When β-hCG levels are higher than 1,500 IU per L (the so-called “discriminatory zone”) with no visualized IUP, there should be a high suspicion for ectopic pregnancy regardless of symptoms.5
Differential Diagnosis
This includes appendicitis, PID, ruptured corpus luteum cyst, ovarian torsion, urinary tract disease, and threatened or incomplete intrauterine abortion. Consider concurrent problems, such as IUP and appendicitis, or IUP and ectopic pregnancy (rare, except in patients who have undergone fertility treatment). Also, rare types of ectopic pregnancy, including interstitial, abdominal, cervical, ovarian, or multiple, should be considered.
TREATMENT
Emergent Presentation
Emergency laparotomy or laparoscopy is indicated if signs of intraperitoneal bleeding or shock are present. Fluids and blood transfusions are given as required. Ultrasonographic examination may waste critical time.
Nonemergent Presentation
• Surgery. Surgical management is favored if pain is prolonged for more than 24 hours, quantitative β-HCG is more than 10,000 IU per L, when fetal cardiac activity is noted, or in ectopics larger than 3.5 cm on ultrasound. Laparoscopy or laparotomy is performed to remove the ectopic pregnancy.5,6
• Methotrexate. Eligible patients for this treatment have a gestational sac diameter less than 3.5 cm, with no fetal cardiac activity, and no evidence of rupture on ultrasound. There is no absolute cutoff for the level of β-HCG in medical management; however, higher β-HCG levels (greater than 5 to 10,000 IU per L) have a higher risk of subsequent rupture.4–6 The current recommended regimen is a single intramuscular injection of the drug at 50 mg per m2. Absolute contraindications include immunodeficiency, liver disease, blood dyscrasia, pulmonary disease, renal dysfunction, or peptic ulcer disease (PUD). The β-HCG level may rise for 3 to 4 days, but should then fall 15% between days 4 and 7. If this does not occur, a second dose of methotrexate or surgical treatment is considered.5
• Expectant. Nearly 47% of ectopic pregnancies can undergo expectant management. Expectant management should be undertaken only in hemodynamically stable patients with β-HCG levels less than 1,000 IU per L and declining. Ectopic mass must be less than 3 cm with no fetal heart rate. Patients electing for expectant management need to be highly reliable, have close follow-up, and be counseled of the risk of ectopic rupture and hemorrhage.
Monitoring of Treatment
β-HCG should be followed weekly until undetectable, regardless of the method of treatment. Rho (D) immune globulin should be given to Rh-negative women.1
SPECIAL CONSIDERATIONS
The mortality rate for ectopic pregnancy in the United States has decreased from 1.15 to 0.50 deaths per 100,000 live births from 1980 to 2007 and continues to decline, largely due to improved detection and timely treatment.7 Medical treatment has been shown to be as efficacious as surgical management in appropriate candidates. Complications from surgery include anesthesia risks, routine surgical risks, postoperative pain, and discomfort. Methotrexate side effects are minimal and self-limiting. The most common are stomatitis and conjunctivitis.
In patients with an ectopic pregnancy, another ectopic pregnancy occurs in 6% to 12% of patients. Patients should be counseled to practice “safe sex” to avoid pelvic infections. Finally, patients should be encouraged to discuss their feelings about the loss of the pregnancy and the possibility of compromised fertility in the future.
REFERENCES
1. Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physician 2005;72:1710–1714.
2. Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol 2006;107(2):399–413.
3. Ankum WM, Mol BW, Van der Veen F, et al. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril 1996;65(6):1093–1099.
4. Bickell NA, Bodian C, Anderson RM, et al. Time and ruptured tubal pregnancy. Obstet Gynecol 2004;104(4):789–794.
5. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 94: medical management of ectopic pregnancy. Obstet Gynecol 2008;111:1479–1485.
6. Goksedef BP, Kef S, Akca A, et al. Risk factors for rupture in tubal ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol 2011;154:96–99.
7. Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States 1980–2007. Obstet Gynecol 2011;117(4):837–843.
| Medical Problems During Pregnancy |
NAUSEA AND VOMITING OF PREGNANCY
General Principles
One of the most common complaints in pregnancy is nausea, affecting almost 85% of women. Another 50% will suffer from vomiting as well. Hyperemesis gravidarum is a severe form of this condition affecting about 1% of pregnant women.1 Nausea and vomiting of pregnancy is most often managed conservatively, but may require hospitalization if symptoms are severe.
Nausea of pregnancy usually starts around 4 to 6 weeks, peaks at 8 to 12 weeks, and subsides by 20 weeks.
Pathophysiology
The mechanism of nausea and vomiting in pregnancy remains unclear, but the clinical course closely follows the waxing and waning levels of human chorionic gonadotropin. A newer hypothesis is that it may be related to Helicobacter pylori.2
Diagnosis
The most important part of the diagnosis of nausea and vomiting of pregnancy is history. Frequency and volume of vomiting as well as any weight loss is important. Migraine headaches and other gastrointestinal disorders should be considered in the differential diagnosis.
Physical examination to assess fluid status is also essential. Important signs are dry mucous membranes, tachycardia, orthostatic hypotension, and poor skin turgor. Weight loss greater than 5% of prepregnant weight, ketonuria, electrolyte abnormalities (hypokalemia), or dehydration (high urine specific gravity) may signify the development of hyperemesis gravidarum. Vomiting of this severity increases the risk of poor fetal outcome.
If hyperemesis gravidarum is suspected, laboratory evaluation should include blood levels of blood urea nitrogen, creatinine, alanine aminotransferase, aspartate aminotransferase, electrolytes, and lipase.
Treatment
Most nausea of pregnancy can be managed conservatively with dietary modifications. Eating small amounts of food several times may be helpful as well as avoiding odors, foods, and supplements that might serve as triggers such as fatty or spicy foods or iron supplements. Randomized controlled trials have not compared different types of diets to control nausea and vomiting in pregnancy.1 About 10% of women with nausea and vomiting in pregnancy require medication therapy. Randomized trials support the use of vitamin B6 (pyridoxine), 10 to 25 mg every 8 hours, and doxylamine, 25 mg at bedtime and 12.5 mg in the morning and afternoon. If symptoms persist, other treatment options include metoclopramide, promethazine, and ondansetron. A randomized controlled trial suggests that metoclopramide give in a dosage of 10 mg every 8 hours is as effective and better tolerated than promethazine for hyperemesis gravidarum.3
APPENDICITIS IN PREGNANCY
General Principles
The most common general surgical problem encountered during pregnancy is acute appendicitis.4 Appendicitis in pregnancy may have an alternative presentation in pregnancy with right upper quadrant pain due to displacement of abdominal contents.
Appendicitis occurs in approximately 1 in 1,500 pregnancies. In one series, 30% of cases occurred in the first trimester, 48% in the second trimester, and 25% in the third trimester.5
Diagnosis
Diagnosis of acute appendicitis can be quite challenging in pregnancy, likely leading to the increased rates of appendiceal rupture. Factors confounding the diagnosis during pregnancy include the relatively high prevalence of abdominal/gastrointestinal discomfort, anatomic changes related to the enlarged uterus, and the physiologic leukocytosis of pregnancy. Pregnant women are less likely to have a classic presentation of appendicitis, especially in the third trimester. Although right lower quadrant pain at McBurney’s point is the most common symptom at any point in pregnancy, pain may also localize to the mid- or upper right quadrant as the uterus enlarges and displaces the bowel.
Compression-graded ultrasonography is the preferred method of imaging the appendix during pregnancy.6 Computed tomography has greater specificity and sensitivity for the diagnosis of appendicitis, but concerns of fetal radiation exposure limit its use to situations in which the clinical findings and ultrasound are inconclusive.
Treatment
Treatment of acute appendicitis is appendectomy. Maternal morbidity following appendectomy is comparable to that in nonpregnant women. The exception is in the case of appendiceal rupture, which significantly increases the rate of fetal loss.7
HYPERTENSION
General Principles
Hypertension is the most common medical problem in pregnancy affecting between 6% and 8% of gestations. There are four categories of hypertension in pregnancy as described by the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy: chronic hypertension, gestational hypertension, preeclampsia, and preeclampsia superimposed on chronic hypertension.8
Hypertension in pregnant women is defined as blood pressure greater than 140/90 measured on more than one occasion. As many women are becoming pregnant at older ages, chronic hypertension now plays a larger role in management issues in pregnant women. Patients should be screened for hypertension at every visit.
Pathophysiology
Most cases of chronic hypertension in pregnancy are caused by essential, or primary, hypertension. This process involves complex hemodynamics and often is associated with obesity. Preeclampsia is associated with abnormal immunologically mediated invasion of the trophoblast into the endometrium. This leads to altered development of placental vasculature. Because the blood flow to the placenta is altered, it releases vasoactive hormones, which cause endothelial dysfunction. In addition, the clotting cascade is triggered by the activated endothelium. These hemodynamic changes lead to hypertension and end-organ damage seen in preeclampsia.
Diagnosis and Treatment
Chronic hypertension is defined as hypertension presenting before 20 weeks gestation or persisting beyond 12-week postpartum. Treatment is not required unless the patient’s blood pressure is persistently greater than 150–180/100 to 110. In this case, treatment with methyldopa, labetalol, or nifedipine should be considered to prevent maternal end-organ damage. Intrauterine growth retardation is of concern in chronic hypertension and should be monitored for accordingly.9
Gestational hypertension, previously termed pregnancy-induced hypertension, describes the development of hypertension after 20 weeks gestation without proteinuria. Management should include close monitoring as 50% of patients will go on to develop preeclampsia. Treatment remains similar to that of chronic hypertension where medications are reserved for severe elevations in blood pressure.
Preeclampsia is a multiorgan disease process of unknown etiology defined as the development of hypertension and proteinuria (greater than 300 mg in a 24-hour urine specimen) after 20 weeks gestation.10 Risk factors for the development of preeclampsia include chronic hypertension, chronic renal disease, obesity, maternal age greater than 40 years, multiple gestation, nulliparity, preeclampsia in a previous pregnancy, and pregestational diabetes mellitus. In cases of mild preeclampsia, expectant management is indicated. This constitutes maternal and fetal monitoring, including blood pressure measurement, laboratory evaluation, nonstress tests, and biophysical profiles along with ultrasound to assess fetal growth. Diagnostic criteria for severe preeclampsia include one or more of the following: resting blood pressure greater than 160 mmHg systolic or 110 mmHg diastolic on two or more occasions, proteinuria greater than 5 g in a 24-hour urine specimen, or any of the associated signs or symptoms (cerebral or visual disturbance, right upper quadrant pain, fetal growth restriction, oliguria, pulmonary edema, thrombocytopenia). Management of severe preeclampsia includes admission to the hospital for complete evaluation of maternal and fetal status. Treatment with magnesium sulfate, antihypertensives, and corticosteroids maybe indicated. Timing of delivery is based on fetal maturity and evaluation of fetal and maternal well-being.
Preeclampsia may progress to eclampsia (preeclampsia with seizure) or HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count). Both of these are immediately life-threatening conditions, which require medical intervention, and plans should be made for prompt delivery.
URINARY TRACT INFECTIONS AND ASYMPTOMATIC BACTERIURIA
General Principles
Urinary tract infections are common in pregnant women. They can be defined as either lower tract (acute cystitis) or upper tract (acute pyelonephritis). Likewise, asymptomatic bacteriuria, the presence of a positive urine culture in a patient without symptoms, is also fairly common in pregnancy, affecting between 2% and 5% of pregnant women. While benign in the nonpregnant woman, asymptomatic bacteriuria in pregnancy does pose a threat to the well-being of mother and fetus. The most common causative agents are Escherichia coli, Klebsiella, Proteus, and Streptococcus group B.
Many physiologic changes lead to an increased tendency for pregnant women to harbor bacteria in their urine. Progesterone can cause smooth muscle relaxation, leading to less ureteral peristalsis and urine stasis. Hormones also cause the bladder to increase its capacity. In addition, mechanical changes such as compression of the ureters by the enlarging uterus and a positional change of the bladder due to shifting of the abdominal organs can also cause urinary stasis. Any stasis of the urine provides a good culture medium for bacteria.11
Diagnosis
Screening for asymptomatic bacteriuria should be performed at 12 to 16 weeks gestation (or at the first prenatal visit).12 Rescreening is generally not performed in low-risk women. Acute cystitis is the presence of bacteriuria of the lower urinary tract with associated signs and symptoms such as dysuria, frequency, hematuria, and lower abdominal discomfort. Patients with acute pyelonephritis generally present with fever, flank pain, and nausea/vomiting, which may occur in the presence or absence of cystitis symptoms.13 Laboratory evaluation should include a complete blood count (CBC) with differential, electrolytes, blood urea nitrogen, creatinine, urine analysis, and urine and blood cultures.
Treatment
For asymptomatic bacteriuria and acute cystitis, treatment options include nitrofurantoin 100 mg BID for 5 days at bedtime for 10 days; amoxicillin 500 mg BID for 5 days; or cephalexin 500 mg BID for 5 days. Urine culture should be repeated after treatment to ensure successful eradication. If the culture remains positive, antibiotic therapy should be adjusted to resistance patterns and duration of therapy increased to 7 to 10 days. Patients with a history of asymptomatic bacteriuria or acute cystitis should continue to be screened for recurrence on a monthly basis for the remainder of their pregnancy. If the patient continues to have recurrences, prophylactic treatment should be given for the remainder of the pregnancy using nitrofurantoin 50 to 100 mg PO at bedtime. Regular repeat urine cultures should be done throughout the pregnancy.
Because of the higher risk of complications, acute pyelonephritis in pregnancy is generally treated with hospital admission and intravenous antibiotics until the patient is afebrile or asymptomatic for at least 48 hours at which point a transition to oral antibiotics can be considered. Preferred antibiotic therapy is parenteral β-lactams. If symptoms and fever persist beyond the first 24 to 48 hours of treatment, imaging should be considered to further evaluate the urinary tract for obstruction or abscess.
Complications
About 30% of women with asymptomatic bacteriuria go on to develop pyelonephritis. Eradication of the bacteriuria reduces the risk of pyelonephritis to about 5%. In addition, treatment of asymptomatic bacteriuria has been shown to reduce the incidence of preterm labor, which is four times higher if untreated, as well as reduce the incidence of low-birth-weight infants. A case-control study of over 15,000 pregnant women found an increased risk of preeclampsia in association with either asymptomatic bacteriuria or symptomatic urinary tract infection.14 Preterm birth is also more common in pregnant women with acute pyelonephritis during pregnancy.15
DIABETES MELLITUS
General Principles
Diabetes mellitus is a serious chronic illness that can have major effects on the outcome of pregnancy. Tight glycemic control is key to avoiding complications. Like hypertension, diabetes in pregnancy can be either pregnancy-induced or pre-existing. Gestational diabetes is defined as diabetes that is first recognized during pregnancy. This means that a patient could have pre-existing type 2 diabetes that is first recognized during routine prenatal laboratory tests. In this case, during the pregnancy, the disorder would be called gestational diabetes and if it continued after the pregnancy it would be reclassified as type 2 diabetes. Gestational diabetes is common (affecting 5% to 9% of pregnancies in the United States and growing in prevalence) and signals patients who are at increased risk of developing type 2 diabetes later in life even if they were euglycemic prior to pregnancy.
Gestational diabetes most often develops in the second or third trimesters, when the placenta begins to secrete hormones that cause increased insulin resistance. It is believed that patients with new-onset diabetes during pregnancy are likely predisposed to insulin resistance and that pregnancy simply pushes them over the edge into insulin resistance and hyperglycemia.
Diagnosis
Screening for gestational diabetes is important, but risk stratification is necessary. Patients who are obese, have a family history of diabetes mellitus, have a personal history of insulin resistance or delivery of a macrosomic infant, or have glucosuria are considered at high risk for developing gestational diabetes. Average-risk patients require screening for gestational diabetes between 24 and 28 weeks, whereas high-risk patients should be screened at the first prenatal visit and again at 24 to 28 weeks.16
Screening starts with a 50-g 1-hour oral glucose challenge. A positive test is a serum glucose measurement of >140 mg per dL after 1 hour. This test does not require fasting. A positive glucose challenge is followed up by a 100-g 3-hour oral glucose tolerance test. First, a fasting glucose level is measured (normal <95). Sequential serum glucose measurements are then taken 1 hour (normal <180), 2 hours (normal <155), and 3 hours (normal <140) after the 100-g dose of glucose. A positive test is determined if two or more of these measurements are above the normal range.
Treatment
First-line treatment for gestational diabetes should begin with dietary medication, often in consultation with an experienced nutritionist. If fasting blood sugars remain greater than 95, or 2-hour postprandial blood sugars remain above 120, pharmacologic therapy should be instituted. Insulin is often considered first-line; however, glyburide has been proven to be safe and effective for use in management of gestational diabetes, and there is increasing evidence to support the use of metformin.
Fetal surveillance is also an important part of the management of gestational diabetes. This may include screening for congenital abnormalities and twice weekly nonstress testing and weekly amniotic fluid index beginning in the third trimester. Timing of delivery remains somewhat controversial and should be based on glycemic control and other risk factors, but there is little evidence to support elective delivery prior to 39 weeks.
All patients with gestational diabetes should be rescreened at 6 to 12 weeks postpartum for persistent diabetes.
Complications
Hyperglycemia early in pregnancy has been shown to cause defects in organogenesis. During the first few weeks of gestation, when organogenesis occurs, the embryo cannot make its own insulin and maternal insulin does not cross the placenta. Thus, maternal hyperglycemia directly leads to hyperglycemia in the developing baby. For this reason, preconception glycemic control is important in diabetic women of childbearing age. Second, maternal hyperglycemia often leads to fetal macrosomia and problems with delivery as well as neonatal hypoglycemia and increased risk of neonatal death.
REFERENCES
1. Barclay L. ACOG guidelines for treating nausea and vomiting in pregnant women reviewed. N Engl J Med 2010;363:1544–1550.
2. Quinlan JD, Hill DA. Nausea and vomiting of pregnancy. Am Fam Physician 2003;68:121.
3. Tan PC, Khine PP, Vallikkannu N, et al. Promethazine compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol 2010;115(5):975–981.
4. Tamir IL, Bongard FS, Klein SR. Acute appendicitis in the pregnant patient. Am J Surg 1990;160:571.
5. Mahmoodian S. Appendicitis complicating pregnancy. South Med J 1992;85:19.
6. Wand PI, Chong ST, Kielar AZ, et al. Imaging of the pregnant and lactating patients: part 2, evidence based review and recommendations. Am J Roentgenol 2012;198:785.
7. Silvestri MT, Pettker CM, Brousseau EC, et al. Morbidity of appendectomy and cholecystectomy in pregnant and nonpregnant women. Obstet Gynecol 2011;118:1261.
8. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183(1):S1–S22.
9. ACOG Committee on Practice Bulletin. Chronic hypertension in pregnancy. Obstet Gynecol 2001;98(1 Suppl):177–185.
10. Davison JM, Homuth V, Jeyabalan A, et al. New aspects in the pathophysiology of preeclampsia. J Am Soc Nephrol 2004;15(9):2440–2448.
11. Cunningham FG, Lucas MJ. Urinary tract infections complicating pregnancy. Bailliere’s Clin Obstet Gynecol 1994;8:353.
12. Lin K, Fajardo K; U.S. Preventative Services Task Force. Screening for asymptomatic bacteriuria in adults: evidence for the U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 2008;149:W20.
13. Hooton TM, Gupta K. Urinary tract infections and asymptomatic bacteriuria in pregnancy. www.uptodate.com. Accessed July 30, 2014.
14. Minassian C, Thomas SL, Williams DJ, et al. Acute maternal infection and risk of preeclampsia: a population-based case-control study. PLoS One 2013;8:e73047.
15. Wing DA, Fassett MJ, Getahun D. Acute pyelonephritis in pregnancy: an 18-year retrospective analysis. Am J Obstet Gynecol 2014;210:219.e1.
16. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth international Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl 2):S251–S260.
|
GENERAL PRINCIPLES
Post-term or postdate pregnancy (PDP) is pregnancy lasting beyond 42 weeks, or 294 days measured from the first day of the last menstrual period.1 The incidence of PDP ranges from 0.4% to 8.1%.2
An accurate estimate of gestational age based on early pregnancy ultrasound with measurement of the crown–rump length has been shown to decrease the incidence of postdate pregnancies when compared with other dating modalities (last menstrual period, and known date of conception).2
DIAGNOSIS
The most common cause of PDP is the inaccurate dating of the pregnancy. Other causes are unclear, but risk factures include previous PDP (50% recurrence rate); family history of PDP; primigravity; excessive maternal weight gain; maternal obesity; male fetal gender; lower socioeconomic status; and fetal abnormalities such as anencephaly, adrenal insufficiency, and placental sulfatase deficiency.2
TREATMENT
Treatment of PDP remains controversial. Expectant as opposed to active management between 41 and 42 weeks yields no statistically significant difference in perinatal or neonatal morbidity. Despite this, expectant management is associated with a statistically significant increased risk for cesarean delivery and meconium aspiration syndrome.2,3 Births after completion of the 42nd week are associated with a slightly increased risk to the infant and are associated with greater number of deaths.3,4
Expectant Management
It is recommended that routine fetal surveillance for PDP begins between 40 and 42 weeks gestation. Despite this, there are no randomized controlled trials (RCTs) demonstrating the efficacy of such monitoring in the reduction of fetal mortality or morbidity. This fetal assessment can take the form of a biophysical profile (BPP), a modified BPP consisting of a non-stress test (NST) with an ultrasound for amniotic fluid index (AFI), or a contraction stress test. Abnormalities in any of these tests may indicate the need for induction. By convention, antenatal testing is started at 41+0 with biweekly NSTs and weekly AFI. By 43 weeks gestation, all patients should be delivered due to the fact that fetal morbidity and mortality generally increase beyond that gestational age.3
Active Management
Active management of the PDP advocates for the induction of labor to reduce perinatal complications. The timing of the induction remains controversial. There are no RCTs comparing induction at 41 versus 42 weeks. The relative risk of perinatal mortality increases between 41 and 42 weeks (0.25 and 0.32, respectively). Resulting from this, it is generally recommended that labor be induced in women without signs of ensuing labor prior to the completion of the 41st week.2 Elective induction of labor is associated with a nonsignificant difference in perinatal mortality compared with expectant management for PDP (relative risk 0.33, 95% CI: 0.10 to 1.09, p = 0.07; 11 RCTs). Elective induction of labor is associated with a significant reduction in meconium aspiration syndrome (relative risk 0.43, 95% CI: 0.23 to 0.79, p = 0.007; seven RCTs), a significantly lower mean birth weight (weighted mean difference −44.41, 95% CI: −79.37 to −9.45, p = 0.01; eight RCTs), and a significant reduction in cesarean section (relative risk 0.87, 95% CI: 0.80 to 0.96; p = 0.004; 13 RCTs). There were no statistical differences in other outcomes for the two groups.5 Women should be informed that number of inductions needed to treat is 500 in order to prevent a single perinatal death and that women who opt for expectant management of PDP may have a higher rate of cesarean delivery than their active management counterparts.
• Cervical ripening agents. Dinoprostone, a prostaglandin E2 (PGE2) analogue, has been shown to improve induction outcome as well as decrease the length of induction time in patients with an unfavorable cervix. Prepidil (gel) in a standard dose of 0.5 mg administered intracervically can be used every 6 hours as needed to a maximum dose of 1.5 mg PGE2 or 7.5 mL PGE2 gel.6 Cervidil (10 mg vaginal insert) is introduced into the posterior fornix of the vagina for 12 hours. Continuous fetal monitoring is recommended during use of either agent. Both agents require refrigeration and are extremely expensive. Misoprostol (Cytotec), a PGE1 analogue, is also used for cervical ripening and labor induction (not Food and Drug Administration approved). Cytotec 25 to 50 μg inserted intravaginally into the posterior fornix used every 3 to 4 hours significantly reduces labor time. Uterine tachysystole and hyperstimulation can occur; therefore, continuous fetal monitoring is recommended. Misoprostol is temperature stable and inexpensive.7
• Membrane stripping or sweeping has also been used as a method for inducing labor. Sweeping membranes somewhere between every other day and once weekly starting at 39 weeks can reduce the number of women who reach 41 weeks gestation. Risks of the procedure include membrane rupture, infection, and bleeding.8
• Amniotomy, with or without oxytocin, is widely used to induce labor. Early amniotomy shortens the duration of labor and reduces the incidence of dystocia but does not reduce the need for anesthesia or cesarean section. Timing of the amniotomy is important because once it is done the patient is committed to delivery. Amniotomy is best performed in conjunction with the administration of some agent to induce contractions, when there are regular uterine contractions and the head is well applied to the cervix.9
• Oxytocin administration remains the most common form of labor induction. Various protocols exist. The use of the low-dose infusion (starting at 1.0 to 2.0 mU per minute and increasing the dose every 15 to 30 minutes with a maximum of 20 mU per minute) is associated with less uterine hyperstimulation, water intoxication, and antidiuretic effect than high-dose protocols. Misoprostol is also being used for labor inductions and has been shown to shorten times to delivery and a significant decrease in cesarean when compared with pitocin but has higher rates of tachysystole and hyperstimulation.10 Fetal monitoring should be continuous during induction to ensure fetal well-being.11
COMPLICATIONS
Expectant Management
Neonatal complications include stillbirth, birth asphyxia, placental insufficiency, umbilical cord complications, bone fracture, pneumonia, septicemia, and meconium aspiration.12 Macrosomia occurs more often (3% to 7% in PDP) and is associated with an increased risk for shoulder dystocia, neurologic injuries to the shoulder girdle, and cephalohematoma. Hypoglycemia is often seen during the neonatal period in the macrosomic infant. Post-maturity syndrome, characterized by peeling skin, calcified skull, and thin body due to wasting of subcutaneous fat, complicates up to 10% of pregnancies between 41 and 42 weeks, and increases to 33% of pregnancies at 43 weeks.2 Maternal complications include an increased incidence of cephalopelvic disproportion, labor dystocia, anxiety, chorioamnionitis, postpartum hemorrhage, vaginal and rectal lacerations, endometritis, and double the rate of cesarean section.2
Active Management
See Chapter 14.10 for complications during induction of labor.
REFERENCES
1. ACOG Committee Opinion No. 579: definition of term pregnancy. Obstet Gynecol 2013;122(5):1139.
2. Simpson PD, Stanley KP. Prolonged pregnancy. Obstet Gyneacol Reprod Med 2011;21:257.
3. Clinical Practice Obstetrics Committee, Maternal Fetal Medicine Committee, Delaney M, Roggensack A, Leduc DC, et al. Guidelines for the management of pregnancy at 41+0 to 42+0 weeks. J Obstet Gynaecol Can 2008;30:800.
4. Gülmezoglu AM, Crowther CA, Middleton P, et al. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev 2012;6:CD004945.
5. Wennerholm UB, Hagberg H, Brorsson B, et al. Induction of labor versus expectant management for post-date pregnancy: is there sufficient evidence for a change in clinical practice? Acta Obstet Gynecol Scand 2009;88(1):6–17.
6. Sawai SK, Williams MC, O’Brien WF, et al. Sequential outpatient application of intravaginal prostaglandin E2 gel in the management of postdate pregnancies. Obstet Gynecol 1991;78:19.
7. Wing D. Labor induction with misoprostol. Am J Obstet Gynecol 1999;181:339.
8. Cammu H, Haitsma V. Sweeping of the membranes at 39 weeks in nulliparous women: a randomized controlled trial. Br J Obstet Gynecol 1998;105:41.
9. Fraser WD, Turcot L, Krauss I, et al. Amniotomy for shortening spontaneous labour (Cochrane review). In: The Cochrane Library, Issue 2:2000. Oxford: Update Software.
10. Induction and augmentation of labor. ACOG Technical Bulletin Number 157—July 1991. Int J Gynaecol Obstet 1992;39(2):139–142.
11. Sulik SM. Postdate pregnancy. In: Taylor RB, ed. Manual of family practice. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002.
12. Olsen AW, Westergaard JG, Olsen J. Perinatal and maternal complications related to post-term delivery: a national register-based study, 1978–1993. Am J Obstet Gynecol 2003;189:222.
| Obstetric Problems During Pregnancy |
PREECLAMPSIA AND ECLAMPSIA
Preeclampsia is potentially life-threatening complication of late pregnancy characterized by hypertension, proteinuria, and multiorgan dysfunction. Preeclampsia is a progressive syndrome that requires frequent reevaluation of both maternal and fetal health. In severe cases, patients may develop abnormalities of the central nervous, hepatic, and hematologic systems, including generalized seizures known as eclampsia.
General Principles
Definitions
Preeclampsia is the new onset of systolic blood pressure (SBP) 140 mmHg or higher and/or diastolic blood pressure (DBP) 90 mmHg or higher after 20 weeks of gestation plus any of the following:1
• Proteinuria (300 mg or more in 24 hours or protein/creatinine ratio 0.3 or higher)
• Thrombocytopenia (platelet count less than 100,000 per μL)
• Impaired kidney function (creatinine more than 1.1 mg per dL or a doubling from baseline creatinine)
• Impaired liver function (transaminases more than twice normal values)
• Pulmonary edema
• Cerebral or visual symptoms
Eclampsia is the occurrence of generalized seizures in a preeclamptic woman; this is one of several severe features of preeclampsia. Other severe features of preeclampsia are shown in Table 14.8-1. Hypertension should be confirmed with two measurements at least 4 hours apart. Severely elevated BP readings may be verified after shorter intervals in order to facilitate timely intervention.
Epidemiology
Preeclampsia complicates 3.1% to 3.4% of pregnancies in the United States. With appropriate treatment, eclampsia occurs in less than 1% of preeclamptic patients. The incidence of eclampsia in the United States and Canada is approximately 5.6 to 5.9 per 10,000 deliveries. Risk factors for preeclampsia include nulliparity, age less than 20 years or greater than 35 years, African American ethnicity, preeclampsia in a previous pregnancy, prolonged interval since previous pregnancy, chronic hypertension, multiple gestation, diabetes mellitus, chronic kidney disease, connective tissue disease, antiphospholipid antibody syndrome, obesity, and family history of preeclampsia.
Classification
Preeclampsia is classified as a hypertensive disorder of pregnancy. However, hypertension is thought to be a sign rather than the cause of the underlying disease process.
Diagnosis
History
At the initial prenatal visit, screen patients for risk factors for preeclampsia, and note complications of previous pregnancies. Beginning at 20 weeks of gestation, assess patients routinely for headache, visual disturbances, abdominal pain, and edema. Preeclampsia usually presents in the third trimester of pregnancy but can occur any time after 20 weeks of gestation, during labor, or even after delivery. Postpartum cases are most likely to occur within 48 hours of delivery.
Symptoms, if present, indicate more severe disease. Neurologic symptoms include headache, visual changes, confusion, coma, hyperreflexia, stroke, and seizures (eclampsia). Visual changes consist of scotomata, diplopia, blurred vision, blind spots, and transient cortical blindness. Gastrointestinal symptoms include nausea, vomiting, and epigastric or right upper quadrant abdominal pain resembling heartburn. Edema of the legs, hands, and face may persist after bed rest. Dyspnea due to pulmonary edema may occur. Preeclampsia can progress rapidly.
Physical Examination
Measure BP at every prenatal visit and monitor weight regularly. Patients with preeclampsia may gain a pound of fluid or more daily. Lower extremity edema is a common finding in normal pregnancy, but edema of the face and upper extremities is more closely associated with preeclampsia. Fundal height measurements that do not match dates may indicate fetal growth restriction (FGR). Assess deep tendon reflexes in patients with preeclampsia.
Laboratory and Imaging
In patients who develop SBP 140 mmHg or higher and/or DBP 90 mmHg or higher after 20 weeks of gestation, measure urine protein excretion by one of the following methods:
• 24-hour urine protein (preferred): 300 mg or more is diagnostic of preeclampsia
• Protein/creatinine ratio on a random urine sample: less than 0.15 is normal, 0.3 or higher is suggestive of preeclampsia
• Urine dipstick protein: 1+ or more (should be verified by quantitative testing)
Measure platelet count, serum creatinine, and liver enzymes both for diagnosis and for assessment of severity. Once a diagnosis of preeclampsia has been established, recheck platelets, creatinine, and liver enzymes at least weekly until delivery to monitor for worsening of disease. Preeclampsia with severe features may require more frequent testing.
Obtain peripheral blood smear and lactate dehydrogenase (LDH) to evaluate for hemolysis. The constellation of hemolysis, elevated liver enzymes (aspartate aminotransferase >70 IU per L), and low platelets (<100,000 per μL), known as HELLP syndrome, is a marker of severe disease. Hemolysis is evidenced by schistocytes on blood smear, LDH >600 IU per L, total bilirubin ≥1.2 mg per dL, or serum haptoglobin ≤25 mg per dL.
Careful monitoring for signs of maternal and fetal compromise is critical for timing of delivery. Fetal surveillance should be performed on the following schedule in preeclamptic women:
• Fetal movement counts daily (more than 10 kicks daily is considered normal)
• Non-stress test (NST) weekly
• Biophysical profile weekly, usually alternated with NST for total of two tests weekly; repeat more frequently for suspected FGR or oligohydramnios or as indicated by severity of maternal condition
• Ultrasound assessment of fetal growth every 3 weeks
• Umbilical artery Doppler velocimetry if pregnancy is complicated by FGR
Differential Diagnosis
Distinguishing preeclampsia from chronic hypertension and gestational hypertension may prove challenging. Preeclampsia can also be superimposed on chronic hypertension. Depending on the presentation, the differential diagnosis for preeclampsia and eclampsia may include hepatitis, acute fatty liver of pregnancy, migraine, stroke, epilepsy, pulmonary embolism, systemic lupus erythematosus, and thrombotic thrombocytopenic purpura–hemolytic uremic syndrome.
Treatment
Behavioral
Ambulatory management is appropriate until 37 weeks for patients with preeclampsia without severe features. Indications for hospitalization include preeclampsia with severe features, disease progression, uncertainty about diagnosis or severity, non-reassuring maternal or fetal condition, and barriers to treatment and follow-up. Bed rest has no proven benefit for preeclamptic patients and may increase the risk of venous thromboembolism. Sodium restriction is not recommended during pregnancy, because it may cause volume depletion.1 Smoking during the third trimester greatly increases the risk of FGR and should be discontinued.
Medications
• Anticonvulsants. Magnesium sulfate should be given to prevent seizures in women who have preeclampsia with severe features (see Table 14.8-1).2 Magnesium also is the drug of choice for eclampsia.1 Start with a 6-g intravenous (IV) bolus followed by a 2-g per hour IV infusion. Check serum magnesium 4 hours later; therapeutic levels are 4.8 to 8.4 mg per dL. Monitor reflexes, respirations, mental status, and urine output. Continue the magnesium infusion for 24 to 48 hours postpartum until the patient is stable and diuresing well. Somnolence, muscle weakness, loss of deep tendon reflexes, bradycardia, and hypotension may herald magnesium toxicity. Toxicity is uncommon in women with normal renal function but can be reversed with IV calcium gluconate.
Severe Features of Preeclampsia |
• Systolic blood pressure ≥160 mmHg and/or diastolic blood pressure ≥110 mmHg on two measurements at least 4 h apart while at rest
• Thrombocytopenia (platelet count <100,000/μL)
• Impaired liver function (transaminases more than twice normal values, severe persistent right upper quadrant or epigastric pain)
• Progressive renal insufficiency (creatinine >1.1 mg/dL or a doubling from baseline creatinine)
• Pulmonary edema
• New-onset cerebral or visual symptoms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







