Evolution of Biofeedback in Physical Medicine and Rehabilitation
Steven L. Wolf
He (Helen) Huang
Biofeedback (BF) can be defined as the use of instrumentation to reveal covert physiological processes via user detectable cues, such as visible light and audible tone, for appropriate response shaping. The BF loop consists of a BF machine and user, and as necessary an instructor/trainer/clinician. Ultimately, the machine functions as an extension of human sense to record and display the internal physiological signals or events; the user, armed with feedback information, acquires the skills to control the physiological response toward desired state through mind-body self-regulation.
Biofeedback became recognized as an alternative or adjunct medical tool in the 1960s and has been applied to psychotherapy, physical medicine, sports medicine, incontinence treatment, pain management, and more recently, in the management of other behaviors associated with pediatrics and oncology. This presentation is directed toward rehabilitation medicine in general and the control of movement in particular.
In physical medicine and rehabilitation, the initial BF application arose from diagnostic and research work in electromyography (EMG) (1), which became the most practical BF form in the field. From the 1960s to the 1990s, most clinical and experimental BF applications used EMG, joint angle, position, force, or pressure to reeducate the control of muscles, joint, and balance in patients with various neuromotor deficits. The outcomes provided concrete evidence that objective neurological signs and symptoms can be altered, particularly in patients with upper motor neuron paralysis and spasticity resulting from brain damage (2, 3, 4, 5). However, the initial BF applications did not correlate well to patients’ motor function improvement. This observation may be partly due to the early “static” BF training paradigm, in which patients received BF training in a static posture or during nonfunctional related movement. Since the mid-1990s, several experimental studies, inspired by the concept of task-oriented training for motor functional recovery, have transformed BF interventions into functional task training that might employ feedback principles (6, 7, 8, 9, 10, 11). In addition, recent technological advances have further promoted this new direction. The emergence of novel sensors, advanced signal processing and control, remote communication, and three-dimensional (3D) displays in BF applications will further leverage the influence of BF therapy in physical medicine and rehabilitation.
The purposes of this chapter are to (a) provide a general review of the early experimental and clinical BF applications in physical medicine and rehabilitation and (b) introduce recent developments in rehabilitation technologies and applications toward task-oriented BF interventions. Readers are encouraged to keep in mind that some of these new technologies and applications are still in their infancy; continuous efforts are demanded to establish effective training protocols and outcome measurements and in evaluating these new BF technologies and interventions before reaching definitive conclusions.
TRADITIONAL BIOFEEDBACK IN REHABILITATION
Methods of Biofeedback
Electromyographic Biofeedback
The electromyographic signal or EMG is an electrical manifestation of muscle activity and an effective window to inspect neuromuscular control system. In BF retraining, EMG is the most used form to down-train hyperactive muscles or up-train flaccid or weak muscles in patients with various sensorimotor deficits (12, 13, 14), thus further improving patients’ control over joint. Usually, bipolar surface EMG electrodes are placed on one or two targeted muscles. The sensed signals are digitally sampled at 1,000 Hz or higher rate. The raw signal, the integrated EMG, or the frequency of EMG is then translated into simple acoustic and visual signals (e.g., lights and audio cues) or graphic computer displays (Fig. 70-1). Patients receive the feedback in a quiet environment and mostly in a static posture. Noise is held to a minimum, and visual distractions avoided. Special uses of intramuscular EMG (IMG) BF include attempts at training deep inaccessible muscles, paralytic muscles, muscles separated from the skin by considerable adipose tissue, or muscles that are not easily isolated by surface electrodes. IMG signals are commonly recorded by invasive, indwelling fine-wire or concentric needles and sampled at 10 kHz or higher rate.
Joint Angle Biofeedback
Joint angle BF can be efficient for improving joint movement control (15), even more than EMGBF (5). When the active joint motion is presented but limited in patients with neuromotor deficits, compared to EMGBF, joint angle BF might
be promising for effective and expeditious recovery of joint control. In addition, angle BF is indicated when the goal of training is the regulation of joint movement, such as correction of genu recurvatum (16,17) or the control of movement with appropriate timing and coordination (5,6). Moreover, joint angle BF may be used when the muscle that must be monitored is inaccessible or difficult to isolate. Electrogoniometers reliably reproduced the clinical measurements of joint angle (11); they have been widely employed in angle BF devices. Other applied sensors include mercury tilt switches and gyroscopes. The quantified joint angle is fed back to the patient during single joint movement for targeted angle tracking (15), or during multijoint coordinated movement, such as gait (6,11).
be promising for effective and expeditious recovery of joint control. In addition, angle BF is indicated when the goal of training is the regulation of joint movement, such as correction of genu recurvatum (16,17) or the control of movement with appropriate timing and coordination (5,6). Moreover, joint angle BF may be used when the muscle that must be monitored is inaccessible or difficult to isolate. Electrogoniometers reliably reproduced the clinical measurements of joint angle (11); they have been widely employed in angle BF devices. Other applied sensors include mercury tilt switches and gyroscopes. The quantified joint angle is fed back to the patient during single joint movement for targeted angle tracking (15), or during multijoint coordinated movement, such as gait (6,11).
Pressure or Force Biofeedback
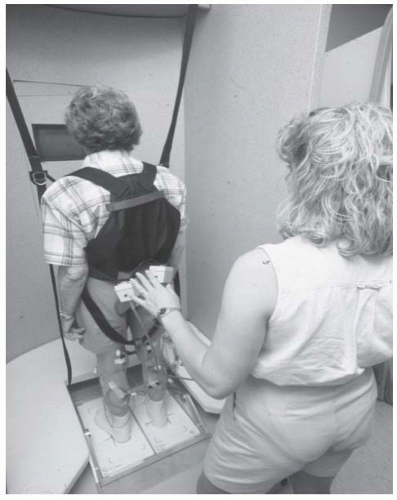
Force or pressure monitoring may be indicated when information concerning the amount of force being transmitted through a body segment or assistive device is desired. Force/pressure sensitive platforms whose applications are described in more detail later in this chapter (see “Balance Rehabilitation”) are often used for retraining of balance (18). As shown in Figure 70-2, a patient with balance control deficits stands on a force plate that measures the ground reaction force or pressure and/or moments in three orthogonal directions under the feet. The derivative of force or pressure measurements such as center of force (COF) (18), center of pressure (COP) (19, 20, 21, 22), or center of gravity (COG) (23, 24, 25) is displayed as a cursor projected on a two-dimensional (2D) screen in front of patients. The goal of the patients is to move the cursor to a desired location or within a targeted area (18). Some commercial force platforms are also equipped with motors that can translate or tilt the platform for balance perturbation (26,27). Force BF training under conditions of posture perturbation have been applied to permit older adults at risk of fall to experience strategies to abort falls (14,24,28). Most of applications feedback the COP cursor instantaneously and such training has been reported to induce both favorable (the amplitudes of the horizontal motion of the COG are diminished) and unfavorable features (the vertical difference between the COG motion and the COP trajectories are enhanced) for reacquisition of balance control (20). Findings from one study suggested that delayed visual feedback of the COP cursor exceeding 600 ms can significantly suppress the unfavorable features and retain the beneficial aspect of COP BF training (20).
Recent sensor technologies, such as insole pressure sensors (29) and portable pressure walkways (30), allow force/pressure BF training in ambulation. All these devices can display the pressure distribution and the COP in real time. In addition to the force measurement using force plates, the loading force or pressure measured by load cells in assistive devices such as canes (31) or prostheses (32) has been used to train patients’ body-weight loading on the impaired limbs during ambulation.
Miscellaneous Techniques
Beyond EMG, joint angle, and force, many other parameters have been monitored for miscellaneous BF applications in
neuromotor rehabilitation. For example, BF of step length (7), knee-to-knee distance (8), and step timing (9) were applied to correct abnormal gait pattern. Trunk acceleration was the BF parameter for stance balance training (33). The applied BF equipment ranged from a simple mirror to expensive motion tracking cameras.
neuromotor rehabilitation. For example, BF of step length (7), knee-to-knee distance (8), and step timing (9) were applied to correct abnormal gait pattern. Trunk acceleration was the BF parameter for stance balance training (33). The applied BF equipment ranged from a simple mirror to expensive motion tracking cameras.
Some studies conducted BF training using multiple data sources. For example, studies coupled EMGBF with joint angle BF and trained stroke patients to control the joint to a desired position by increasing the recruitment of agonist and/or reducing the muscle activity from antagonist (6,14). The risk in using multisource BF is that multiple quantified feedback cues might overload patients’ perception and confuse patients; careful design of feedback cue displays is essential for successful BF applications.
Biofeedback Modalities
Visual Feedback
Visual displays available with BF devices include banks of lights, liquid crystal display (LCD), meters, oscilloscopes, or computer monitors. The visual display can be binary (0/1 in value or light on/off), digital (integral numbers), or continuous (signal waves or value bar).
The sensitivity scale in the visual display should be determined by the goals for the BF training. In EMGBF, if the EMG electrodes are properly applied, it is theoretically possible to obtain accurate readings of activity as low as 1 µV, but such low integrated levels are important only during generalized relaxation training. Retraining patients with musculoskeletal weakness (e.g., the quadriceps after meniscectomy) requires a feedback device with comparatively low sensitivity settings because such patients often learn to generate several hundred microvolts of integrated EMG during isometric or isokinetic contractions. Most contemporary feedback displays have software that adjusts the range of sensitivities as the patient changes his or her muscle output capability with an intent of providing a continuous range that can be visualized and manipulated by the patient within his or her training sessions.
Caution must be exercised when trainees have visual deficits secondary to brain injury. Furthermore, when the BF training involves activities such as gait, visual feedback should be avoided or kept minimal because vision is largely occupied to guide motor coordination.
Audio Feedback
Many commercially available devices offer auditory feedback in the form of a tone, buzzer, click, or a combination of these possibilities. Similar to visual feedback, the audible feedback could be binary, discrete, or continuous. In devices with binary display, a monotone buzzer is heard only when the patient achieves a specific feedback value preset by the therapist. In EMGBF, a low threshold setting may be used in training for recruitment of activity above a given level in a weak or paretic muscle. Once the patient reliably exceeds this level, the threshold is raised progressively. This technique is often referred to as shaping. The reverse shaping strategy to reduce integrated EMG levels (i.e., reduction of resting hypertonus) also may be used. Additionally, binary auditory feedback is useful for BF therapy set in the activities of daily living (ADL); the auditory tone is given only if needed so that users can concentrate on the daily activities most of the time. The device with discrete audible feedback maps more than two physiological states into sounds with different pitch, duration, or loudness. The continuous auditory feedback directly displays the sampled physiological signal. For example, surface EMG was directly transformed into a sound. When the muscle is activated, the audio components increase in intensity and pitch, which can be resolved by patients as effective feedback required to regulate the muscle activation level.
A number of studies compared the influence of auditory display to that of visual feedback during BF training. The auditory cue was reported to have more patient acceptance than the visual feedback when the BF training was carried out in ambulation (11). Another study found that audio BF had larger effects on reducing COP displacement while visual BF had larger effects on reducing trunk sway, which suggests that the two BF modalities may encourage a different type of posture sway strategy (34).
Tactile Feedback
Applied tactile sensation arises from a simple mechanical vibrating stimulator attached to the skin. By modulating the vibration frequency and amplitude, the vibrating stimulator feeds back the sensed physiological signal to the user (35,36). Vibrotactile feedback is safe, frees the user from having to maintain visual attention to the feedback cues, and presents minimum distraction to others. However, results from previous studies indicate that while the user could easily adapt to the tactile stimulation, the tactile biofeedback cues were ignored as participants engaged in ADL (36). These observations may have resulted from the relatively smaller number of reported studies using tactile BF compared to the use of visual or auditory cues.
Scientific Basis of Biofeedback
The mechanisms underlying successful use of BF are still unclear. In physical rehabilitation, BF may enhance sensorimotor integration because this approach highlights utilization of sensory cues that inform patients about consequences of their movements while allowing them to develop adaptive strategies for motor learning and recovery.
Neurological Basis of Biofeedback
Wolf and Binder-MacLeod (37) have asked, “How do patients process feedback information? What factors account for the patient’s ability to gradually become less dependent upon artificially induced information signals after failing to achieve significant gains using conventional rehabilitation procedures?” Previous studies demonstrated that the BF therapy was associated with cortical reorganization (38,39). Specifically, fMRI studies have shown that following biofeedback training to enhance ambulation following stroke, enhanced activation during
controlled knee flexion extension was seen in the ipsilesional primary sensorimotor cortex. The extent to which such changes are manifest with biofeedback neuromuscular reeducation of different joints and for different diagnoses requires considerable investigation. The two possibilities suggested many years ago by Basmajian (12), either an auxiliary feedback loop to recruit existing cerebral and spinal pathways or the development of new pathways, are worthy candidates for further study.
controlled knee flexion extension was seen in the ipsilesional primary sensorimotor cortex. The extent to which such changes are manifest with biofeedback neuromuscular reeducation of different joints and for different diagnoses requires considerable investigation. The two possibilities suggested many years ago by Basmajian (12), either an auxiliary feedback loop to recruit existing cerebral and spinal pathways or the development of new pathways, are worthy candidates for further study.
Nonetheless, the central nervous system uses a multitude of internal modulatory networks. The role of somatosensory or other subcortical areas, and the basal ganglia should not be underemphasized; their timely activation make voluntary movements meaningful. Proprioception, tactile, auditory, and visual inputs are harmonized into the controls, as is the cerebellum. Therefore, damage resulting from external and internal trauma to area 4 of the cerebral cortex may spare pathways that are primarily engaged for reacquisition of skilled movements, or they may spare redundant pathways that EMGBF training can bring into play. Wolf (40) has further speculated three detailed conceptualizations: (a) override: visual or audio feedback may activate the somatosensory cortex by entering at a level higher than the level of damage; (b) bypass: an appropriate feedforward system can be established via the brain stem motor nuclei; (c) repetition with existing neural circuitry: central synapses previously unused in executing motor commands may be activated by visual and audio feedback. As such, continued training may establish new sensory engrams and help patients perform tasks without feedback.
Plasticity and relearning by the brain should be thought of as a more complex phenomenon than usurping function by specialized areas of the neighboring cortex. There is evidence from rat stroke models that changes in dendritic architecture or density of synaptic boutons are associated with motor relearning, provided that the task practice is repetitive and challenging (41,42). Whether the BF training employs and in turn reinforces these new neural connections is unclear.
Placebo Effect
Basmajian (43) addressed placebo factors that are powerful in all therapies, including BF alleged “cures.” Therefore, the placebo effect needs to be understood, accepted wisely and gratefully, and rebuffed. In fact, many clinical studies, especially those based upon pharmacological interventions, question the impact of the intervention if improvements are not in excess of 30% beyond control values or have not been powered adequately to determine a “minimally clinical important difference” (44,45). A deliberate placebo may be best used in double-blind research studies, or rarely as a therapeutic test.
Biofeedback Applications in Physical Medicine and Rehabilitation
Stroke Rehabilitation
A major application of biofeedback in rehabilitation hospitals and outpatient clinics lies in the treatment of patients following stroke. The National Institute for Health (46) lists stroke as the number one cause of adult disability in the United States. Approximately 780,000 people experience a new or recurrent stroke each year (47). Motor dysfunction after stroke may be characterized by muscle weakness, abnormal muscle tone, abnormal movement synergies, and lack of coordination during voluntary movement. Restoring the neuromuscular control in patients with stroke is essential to further improve their motor functions. EMGBF is a useful tool for reeducating neuromuscular control in stroke rehabilitation (3,4,48, 49, 50, 51, 52). Other forms of BF, such as force and joint angle BF, have also been applied, but with less frequent use.
EMG Biofeedback for Upper- and Lower-Extremity Rehabilitation
Wolf et al. (37,53) investigated patient characteristics that may affect the outcome of EMGBF training of hemiplegic patients. The presence of proprioceptive loss appeared to diminish the probability of making functional gains in the upper limb. Age, gender, hemiparetic side, duration of stroke, previous rehabilitation, and number of training sessions did not have a significant effect; patients should not be excluded from feedback training based solely on either age or length of time since their stroke. However, the optimal time for introducing BF appears to be the same as for any rehabilitation efforts: fairly early.
In EMGBF, a practically standard training scheme has been adopted. Detailed EMGBF training protocols for stroke rehabilitation can be found elsewhere (54). Scientists at Emory University have conducted a series of studies to implement EMGBF for stroke rehabilitation. The belief that neurological patients should first have hyperactive muscle down-trained motivated the application of EMGBF to spastic muscles initially. Relaxation training was applied on patients when they were at rest, during passive movements, during distractive movements, and then during shortening contractions. Once the muscles were relaxed, attention was directed toward the antagonist muscles, the ones that are usually weak and need to be up-trained in EMGBF. Last, the coordination of both muscle groups must be trained for efficient joint control. Ultimately, this BF training paradigm was put into a functional context. While this paradigm did yield significant improvements (3,4,55,56), the greatest predictor for ultimate success in applying EMGBF was in the patients’ abilities to demonstrate small amounts of active extension at the elbow, wrist, and fingers (14). In fact, these observations served as the inclusion criteria for the first forced use and constraint induced movement therapy studies (57, 58, 59, 60).
EMGBF for upper-extremity rehabilitation starts from the shoulder joint, followed by elbow, wrist, and hand (54). EMGBF training of pronation and supination of the forearm is difficult because of the cross talk between the EMG activity from the pronators and supinators and other EMG present in the forearm; EMGBF may be combined with angular BF in cases of severe spasticity or flaccidity. Targeted training of the lower limb is simpler than that of the upper limb. Training of the lower limb need not follow the proximal-to-distal progression for the upper limb. The primary functional goals are improved ambulation and to develop a relatively limited
number of stereotyped patterns used during ambulation. One of the important areas of EMGBF training was for the treatment of foot drop caused by paralysis of the ankle dorsiflexors and spasticity of the plantar flexors (2,61). Other training protocols involve training of multiple joints simultaneously to coordinate, such as the training of hip and knee extension critical for the gait stance phase, hip flexion with knee extension important during the terminal swing phase of gait, and hip extension with knee flexion (54).
number of stereotyped patterns used during ambulation. One of the important areas of EMGBF training was for the treatment of foot drop caused by paralysis of the ankle dorsiflexors and spasticity of the plantar flexors (2,61). Other training protocols involve training of multiple joints simultaneously to coordinate, such as the training of hip and knee extension critical for the gait stance phase, hip flexion with knee extension important during the terminal swing phase of gait, and hip extension with knee flexion (54).
The current view that the task-oriented repetitive training is essential for motor functional recovery motivates clinicians and scientists to conduct BF training when patients are walking (6, 7, 8,17,62, 63, 64). BF retraining during ambulation focuses on specific gait timing, because the coordination of muscles or joints depends on gait phase. In this case, a gait event detection system such as footswitch is essential (6). Continuously monitoring the gait performance, such as gait symmetry, weight loading, or muscle recruitment has also been reported (7,62). Auditory warning buzz provides discrete feedback only if the monitored parameter is out of the desired range. The clinical effect of BF training for stroke rehabilitation is still controversial, the detail of which is summarized later in this chapter (see “Randomized Controlled Trials and Meta-Analyses”).
Balance Rehabilitation
Another major application of BF in stroke rehabilitation is the training of balance control (23,25,65, 66, 67, 68). Roughly 40% of stroke patients will experience a serious fall within a year after having a stroke (69). Unsteadiness during stance, asymmetric weight loading, and decreased ability to move within a weight-bearing posture have been reported among stroke survivors. Nichols gave a thorough review and update on force platform BF for balance retraining (18). The training protocols address three components of the function: steadiness, symmetry, and dynamic stability. In retraining of postural steadiness, stroke patients stand on a force plate, wearing a fall arrest harness. Patients are required to keep the cursor representing COF or COP within a narrow range while they sway the body weight. To improve the control of postural symmetry, the force BF training progresses from static standing to dynamic movements, such as sit-to-stand transfers and stepping in place. The percentage of weight bearing on the nonparetic and paretic leg is quantified and displayed visually and audibly (19,65,70). The goal of trainees is to equalize the weight loading on each leg. The training of postural steadiness and symmetry resulted in the reduction in weight-bearing asymmetry (65,70, 71, 72); however, stroke patients failed to reduce their spontaneous sway amplitude and did not improve on functional measures (65,70). The dynamic postural control will be last trained. The patients are instructed to voluntarily move the COP/COF cursor from one target to another in different directions accurately without falling (21,23). The capability to transfer body weight and adopt a different stance position is a prerequisite for safe mobility (73). Hence, training of dynamic stability is thought to have important links to the function (18), but no strong evidence has been found to support this contention.
Spinal Cord Injuries
Spinal cord injury (SCI) results in varying degrees of weakness and sensory loss at and below (i.e., caudal to) the site of injury. The motor deficits may be represented as muscle weakness and limb paralysis, spasticity, lost of normal bladder control, and breathing difficulty.
The primary goals for interfacing patients with SCI with EMGBF are much the same as those outlined previously for stroke patients. First, attempts are made to reduce hypermotor responses to induced length changes in spastic muscles. Such hyperactive behavior of spastic muscles may occur during spontaneous episodes of clonus or during induced clonic seizures, when the lower or upper extremity responds to various tactile stimuli. Once the patient can reduce such responses in supine, sitting, and ultimately standing postures, efforts are directed toward recruitment of weak muscles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree