Diagnosis
Definition
Example
Tissue injury of unknown origin
Diagnosis of exclusion
Negative tissue and/or blood culture
Joint effusion
Transient synovitis of the hip
Superficial abscess
Superficial to deep fascia of limb or located in hand or foot
Septic pre-patellar bursitis
Superficial forearm abscess
Septic joint
Limited to joint space only with no extension to the surrounding muscles or bone
Synovial aspirate is grossly purulent, >50,000 cells, positive gram stain and/or positive bacterial culture
Septic knee
Osteomyelitis
Isolated to bone only, no extension to sub-periosteal space or surrounding muscle/joint
Proximal femur osteomyelitis
Deep abscess/Pyomyositis
Deep to fascia of limb
Isolated to muscle only, no extension into nearby bone or joint
May include multiple muscle groups
May be mild (edema only), moderate (Phlegmon) or severe (abscess)
Obturator internus myositis
Adductor and rectus femoris myositis
Complex
Involving a combination of bone, muscle and joints
Subperiosteal abscess, pericapsular myositis with ischial osteomyelitis, clavicular osteomyelitis with supraclavicular abscess
Children with MSKI typically present with a constellation of symptoms including limp or inability to bear weight and pain. Physical examination will often reveal joint irritability, pain with ROM, and tenderness to palpation. The workup of pediatric MSKI generally includes baseline laboratory work including blood culture, complete blood count (CBC) with differential, c-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Plain films should also be obtained in order to evaluate for possible trauma. Further work-up is directed by the clinical picture and level of concern by the treating physician and may consist of advanced imaging (ultrasound (US), computed tomography (CT), and/or magnetic resonance imaging (MRI)) to evaluate the location and extent of infection. Some pediatric MSKI can be treated with antibiotics alone, while others require surgical debridement. Early recognition and appropriate treatment leads to favorable outcomes in most cases. Infection is deemed to be disseminated when there are multiple positive blood cultures, multiple foci of infection, evidence of thrombus formation (deep vein thrombosis (DVT) or pulmonary embolism (PE)), septic pulmonary emboli or endocarditis. These children have the potential to become very sick and may require intensive care. While long-term morbidity from pediatric MSKI is low, there are recognized complications from delayed diagnosis and treatment. The two most common sequelae are joint destruction and physeal arrest, which can lead to limb length discrepancy or angular deformity.
Septic Arthritis
Diagnosis
Most children with septic arthritis will demonstrate joint irritability with limited ROM, refusal to bear weight, fever, elevated inflammatory markers such as CRP, ESR, and white blood count (WBC) and joint effusion on ultrasound [1–14]. Other pediatric MSKI can mimic septic arthritis, especially when involving the hip joint. While “Kocher Criteria” has long been used to differentiate between children with septic hip arthritis and transient synovitis, recent studies have shown that this diagnostic criteria is unable to distinguish been septic arthritis and other types of infection (osteomyelitis and pyomyositis) around the hip [15]. For this reason, it has been advocated that in cases where there is concern for septic arthritis of the hip based on clinical exam and elevated inflammatory markers that an MRI be obtained as quickly as possible to evaluate the hip and surrounding structures for contiguous infection. One study of 53 patients evaluated in the emergency room for “rule out septic hip arthritis” demonstrated that pelvic pyomyositis was twice as common as isolated septic hip arthritis based on MRI [15] and a different study found that 70/103 patients with septic arthritis had contiguous osteomyelitis on MRI [16]. Rosenfeld et al. found five variables (age above 3.6 years, CRP > 13.8 mg/L, duration of symptoms >3 d, platelets <314 × 10 cells/μL, and ANC > 8.6 × 10 cells/μL) to be predictive of adjacent infection. Patients with ≥3 risk factors were classified as high risk for septic arthritis with adjacent infection (sensitivity: 90 %, specificity: 67 %, positive predictive value: 80 %, negative predictive value: 83 %). The authors of this study recommend preoperative MRI in patients in who meet ≥3 of these criteria [17]. In cases where there is infection adjacent to the joint in question, MRI also has the added benefit of directing the approach for joint aspiration (Fig. 42.1). Hip joint aspiration is commonly performed through a medial approach, just posterior to the adductor longus tendon. It is known, however, that a large percentage of cases of pelvic pyomyositis affect the adductor musculature. Therefore, there is a theoretical risk of contaminating the hip joint if joint arthrocentesis is unknowingly performed through the standard medial approach [15]. In many institutions, however, MRI is not readily available and in younger patients requires sedation. Therefore in a patient for whom the exam is clearly consistent with septic arthritis or who is becoming septic, treatment can be based on the presence of effusion on US and should not be delayed in order to obtain advanced imaging.
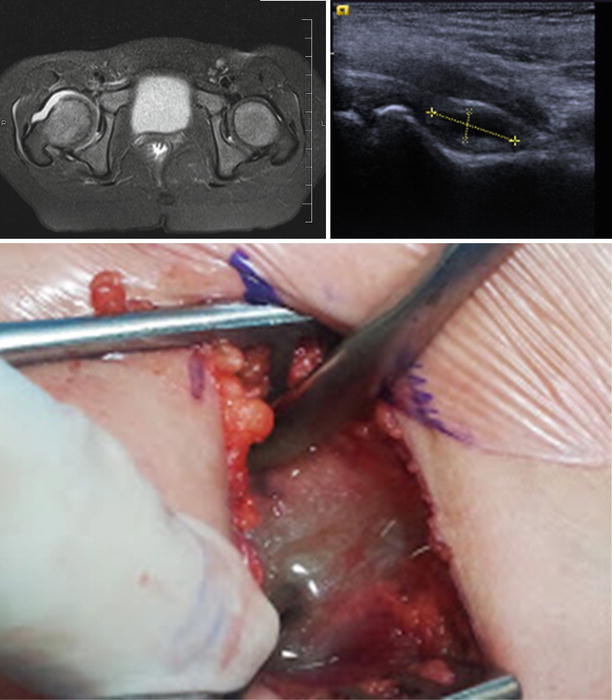

Fig. 42.1
Septic arthritis of the hip joint
The gold standard for diagnosis of a joint infection is the acquisition of a joint fluid sample for gram stain, culture, cell count and occasionally, molecular testing [1, 2, 18]. This can be done under conscious sedation in the emergency department or general anesthesia in the operating room. In most children with bacterial arthritis, the nucleated cell count of the joint fluid aspirate is greater than 50,000 cells/mL with >75 % of the cells identified as segmented neutrophils. Occasionally arthrocentesis can lead to diagnostic uncertainty due to intermediate joint fluid cell counts (10,000–75,000 cells/mL) [19]. In this case, the clinician may consider arthrotomy or arthroscopy as a surgical intervention to treat the possibility of septic arthritis due to incomplete evidence. The most common cause of septic arthritis in children is Staphylococcus aureus and Streptococcus.
In endemic communities, Lyme arthritis due to Borrelia burgdorferi, may demonstrate an overall clinical impression of possible septic arthritis and should be differentiated by supplemental tests [20–23]. This is commonly performed using a sensitive enzyme immunoassay or immunofluorescent assay followed by a Western immunoblot. Joint aspiration is recommended in order to rule out bacterial septic arthritis. Joint fluid cell counts in cases of Lyme arthritis may demonstrate a spectrum of findings [19, 23]. For example, the average cell count for a patient with Lyme arthritis is 60,200 cells/mm3, however, for septic arthritis the average cell count is 123,000 cells/mm3 [23]. In a study of 506 patients with joint effusions, 49 % of the children who were ultimately diagnosed with Lyme arthritis had a joint fluid cell count >50,000 cells/mm3, while 13 % had counts >100,000 cells/mm3. Based on this study, patients with synovial fluid cell counts >100,000 cells/mm3 were more 3.63× more likely to have septic arthritis than Lyme arthritis [23].
Kingella kingae is a gram-negative organism that is becoming increasingly recognized as a common cause of MSKI in children age 6 months to 4 years [24–27]. The clinical presentation of MSKI caused by K kingae is more subtle, with normal or only mildly elevated inflammatory markers. The hip joint is a common site of K kingae infection and therefore a high index of suspicion in needed to make the diagnosis, as these cases are often mistaken for transient synovitis of the hip and blood/synovial fluid cultures are never obtained [24, 25, 28–31]. The benign clinical presentation along with the observation that several patients with culture proven K kingae septic arthritis improve without antibiotic treatment [32] calls into question the importance of missing a septic arthritis caused by K kingae [24]. There have been a few case reports of long term adverse sequelae from K kingae MSKI, which indicates that appropriate antibiotics should be given to all children from whom this organism is recovered [24, 25, 33–35]. K kingae is notoriously difficult to culture from synovial fluid using normal culture techniques. Inoculation of synovial fluid into blood culture vials can significantly improve the recovery of this organism and more recently, real time polymerase chain reaction (PCR) assays have become available to further enhance the detection of K kingae in synovial fluid [24, 36–43]. While PCR shows improved rates of detection of K kingae over traditional joint fluid culture, the turnaround time for results is quite delayed, with an average of 14.6 days at one center [43]. PCR also does not provide any information regarding antibiotic sensitivity [43].
Treatment
Adverse sequelae among children with septic arthritis are well described and are attributed to delayed or inadequate treatment. Clinicians should therefore treat bacterial arthritis by urgent irrigation and drainage of the infected joint [44–55]. Favorable outcomes are generally achieved as long as surgical and antibiotic therapy are initiated for bacterial arthritis within 5 days of the clinical onset of infection [48, 49, 54, 56]. Septic arthritis of the shoulder and elbow in children are also associated with delay in recognition and intervention and therefore require a higher index of suspicion [57, 58]. Successful treatment of bacterial arthritis can be accomplished by either arthrotomy or arthroscopy depending on the surgeon’s level of comfort with these techniques [59–63]. Following irrigation and debridement of the joint, a drain may be placed to allow continued evacuation of the joint space and is typically removed at bedside 2–3 days later. Primary closure of the wound is associated with shorter hospital stays with no adverse affect on outcomes [64]. While aspiration and lavage of the infected joint may act as a temporizing measure, it is recommended that formal irrigation and debridement of the involved joint be performed after bacterial arthritis has been confirmed, unless the clinical and laboratory improvement are substantial [56, 65]. Serial aspirations of bacterial arthritis are rarely performed due to the logistical challenge of repeatedly aspirating joints in children [65]. Children who do not demonstrate appropriate clinical and laboratory improvement within the first 96 h after joint irrigation and debridement may require repeat surgical intervention. The possibility of contiguous osteomyelitis or pyomyositis should be considered and can be evaluated using MRI prior to repeat surgery.
Levels of Evidence
The majority of literature on pediatric septic arthritis are Level III retrospective reviews, with few well designed prospective studies available (Table 42.2). There are also a significant number of case series with Level IV evidence. Based on the best available literature, septic arthritis is best diagnosed based on clinical exam (fever, refusal to bear weight and joint irritability) in combination with several inflammatory markers, including WBC, CRP, and ESR. CRP has the highest sensitivity and specificity. While children with septic arthritis have joint effusions on US, the mere presence of an effusion alone, in the absence of other clinical signs and symptoms of infection, can be a false positive (as in transient synovitis or Lyme arthritis). MRI is slowly replacing US as a better diagnostic test due to the ability of MRI to demonstrate the presence of contiguous infection. The gold standard for diagnosis of septic arthritis remains a culture positive joint aspirate. While synovial fluid cell counts >50,000 cells/mm3 are often considered diagnostic of septic arthritis, there is no good evidence to support this number. There is a wide range of synovial fluid cell counts reported in cases of culture positive septic arthritis, as well as in cases of transient synovitis and Lyme arthritis, making this test difficult to interpret. Clinicians should have a high index of suspicion for K kingae and Lyme arthritis in the appropriate patient populations. K kingae is difficult to culture via standard culture methods and detection of this bacteria is enhanced with the addition of real-time PCR. All available literature recommends early arthrotomy and irrigation of septic joints in children to avoid devastating long term sequelae. There are no studies looking at the optimal timing of such intervention. Several studies, including the lone prospective randomized control trial (Level 1B evidence) suggests that arthroscopic debridement is as effective as open arthrotomy and drainage for the management of septic arthritis in children.
Table 42.2
Levels of evidence for pediatric acute hematogenous osteomyelitis
Author | Type of study | Level of evidence | Grade |
|---|---|---|---|
Kan et al. [67] | RR | III | B |
Ju et al. [102] | RR | III | B |
Shrader et al. [103] | RR | III | B |
Section et al. [104] | RR | III | B |
Gwynne-Jones et al. [68] | CS | IV | C |
Saavedra-Lozano et al. [69] | RR | III | B |
Hawkshead et al. [70] | RR | III | B |
Vander Have et al. [71] | CS | IV | C |
Gafur et al. [72] | RR | III | B |
Creel et al. [105] | CS | IV | C |
Yamagishi et al. [106] | CS | IV | C |
Hulten et al. [73] | PS | IIB | B |
Kaplan et al. [81] | PS | IIB | B |
Fergie et al. (2007) | RR | III | B |
Naimi et al. [75] | PS | IIB | B |
Herold et al. [76] | RR | III | B |
Jungk et al. [77] | RR | III | B |
Frederiksen et al. [107] | CS | IV | C |
Ish-Horowicz et al. [108] | CS | IV | C |
Wong et al. [109] | CS | IV | C |
Goergens et al. [110] | CS | IV | C |
Buckingham et al. [78] | RR | III | B |
Martinez-Aguilar et al. [79] | RR | III | B |
Gonzalez et al. [80] | CS | IV | C |
Kaplan et al. [81] | PS | IIB | B |
Zaoutis et al. [111] | RR | III | B |
Arnold et al. [112] | RR | III | B |
McCaskill et al. [83] | RR | III | B |
Browne et al. [84] | RR | III | B |
Zimbelman et al. [85] | RR | III | B |
Frank et al. [88] | RR | III | B |
Sattler et al. [89] | PS | IIB | B |
Martinez-Aguilar et al. [90] | RR | III | B |
Mishaan et al. [91] | PS | IIB | B |
Chang et al. [92] | RR | III | B |
Seal et al. [93] | CS | IV | C |
Tanir et al. [94] | RR | III | B |
Pannaraj et al. [95] | RR | III | B |
Hasty et al. [96] | RR | III | B |
Olesevich et al. [97] | RR | III | B |
Tsuji et al. [98] | RR | III | B |
Elliott et al. [99] | RR | III | B |
Carrillo-Marquez et al. [100] | RR | III | B |
Young et al. [101] | CS | IV | C |
Hidayat et al. [113] | PS | IIA | B |
Lundy et al. [114] | CS | IV | C |
Trobs et al. [115] | CS | IV | C |
Danielsson et al. [116] | CS | IV | C |
Perlman et al. [117] | CS | IV | C |
Aigner et al. [118] | RR | III | B |
Unkila-Kallio et al. [119] | PS | IIB | B |
Unkila-Kallio et al. [120] | PS | IIB | B |
Roine et al. [121] | RR | III | B |
Khachatourians et al. [122] | RR | III | B |
Arnold et al. [82] | RR | III | B |
Roine et al. [123] | RR | III | B |
Paakkonen et al. [124] | PS | IIB | B |
Copley et al. [125] | RR | III | B |
Courtney et al. [126] | RR | III | B |
Kan et al. [127] | RR | III | B |
Tuason et al. [128] | RR | III | B |
Chou et al. [129] | CS | IV | C |
Paakkonen et al. [130] | CS | IV | C |
Peltola et al. [131] | PSRC | IB | B |
Peltola et al. [132] | PSRC | IB | B |
Peltola et al. [133] | PSRC | IB | B |
Peters et al. [135] | CS | IV | C |
Osteomyelitis
Diagnosis
Children with acute hematogenous osteomyelitis (AHO) may present similarly to those with other osteoarticular infections such as septic arthritis and pyomyositis. On clinical exam, they may have fever and exhibit refusal to bear weight, but typically lack the joint irritability of children with bacterial arthritis. Instead, they may have point tenderness to palpation of the affected bone. Inflammatory markers, including CRP, ESR, and WBC, will be elevated as in other musculoskeletal infections. The best imaging modality to diagnose AHO is MRI, which will demonstrate increased signal intensity within bone on T2 images, but decreased signal intensity on T1 sequences. MRI also has the advantage of also demonstrating contiguous sources of infection, such as sub-periosteal abscess, septic arthritis and pyomyositis [66]. While gadolinium should not be routinely used to evaluate for MSKI in children, contrasted MRI can increased the detection of small abscesses. In a study of 90 children who had MRI with and without contrast performed for evaluation of MSKI, 8 had abscesses requiring surgical intervention that were only identified on post-contrast images. No child with a normal pre-contrast study ended up with a diagnosis of MSKI [67]. Due to the increase in the prevalence of MRSA MSKI in some regions over the last two decades [68–101], one institution created a predictive algorithm to distinguish MRSA vs. MSSA AHO in children. Risk factors for MRSA AHO included hematocrit <34, WBC >12, CRP >13 mg/L and temperature >38. If all 4 factors are present, the risk of MRSA AHO is 92 % [102]. This algorithm, however, has been tested at other institutions with a higher incidence of MRSA infections and was found to have poor diagnostic performance, calling into question the utility of the predictive algorithm outside the original authors’ institution [103].
Treatment
Antibiotics are considered the first line of therapy in children with AHO who have no evidence of abscess formation (intra-osseus, sub-periosteal, or extra-periosteal) on imaging and in whom there is no concern for developing sepsis. The timing of appropriate empiric antibiotic administration should balance the priorities of identifying the causative organism and avoiding the unnecessary delay of antibiotic administration. In a study of 860 children with pediatric MSKI, antibiotic exposure, either pre-hospital or within the authors’ own institution, was not associated with a lower rate of culture positivity of material from the site of infection [104]. Antibiotics should therefore be held until blood cultures have been obtained and given any time thereafter depending on the clinical situation. Whenever there is clinical concern about the potential for disease dissemination or evolving sepsis (high fever, ill appearing, hemodynamic instability, severely elevated inflammatory markers), antibiotics may be given regardless of the timing of advanced imaging or surgery. If urgent aspiration or surgical debridement is planned after initial evaluation or advanced imaging, antibiotics may be held until local tissue culture is obtained or can be started once the decision is made to pursue conservative treatment without surgical intervention or obtainment of local tissue culture. Anaerobic, fungal and AFB cultures should not be routinely performed during the initial evaluation of children with MSKI unless there is a history of immunocompromise, penetrating injury or failed primary treatment [104].
Empiric antibiotic therapy for children with MSKI is dependent upon the local microbiology of the community and surveillance monitoring must be done to ensure appropriate empiric antibiotic selection [68–77, 81, 105, 106]. Neonatal deep infection deserves special consideration as these infections are often acquired in the NICU while the newborn is exposed to invasive lines and catheters [107, 108]. Thus, neonates are at risk of exposure to hospital acquired pathogens, including multi-drug resistant methicillin resistant staphylococcus aureus (MRSA) and Candida Albicans [107, 108]. Neonates who are otherwise healthy and leave the hospital after birth may develop deep infection within a few weeks of birth [45, 109]. These neonates are at risk for infection due to Enterobacteriaceae or group B Streptococcus (GBS) to which they were exposed during delivery. Empiric antibiotic selection in the neonate should therefore cover gram negative organisms, GBS and S aureus [37, 45, 109, 110]. Outside of the neonatal group, the most common causative organism is S aureus [68–77, 81, 105, 106]. MRSA is now thought to be responsible for a sufficient percentage of culture positive cases of AHO in some regions to warrant clindamycin or vancomycin as the empiric antibiotic of choice in children [68, 69, 72–81, 83–101, 111, 112]. In some communities, there is increasing evidence of clindamycin resistance, which typically invokes the use of vancomycin [73]. Monitoring of local antibiotic resistance patterns is therefore essential to ensure that empiric antibiotic therapy is rational based on the local microbiologic epidemiology of the community. When vancomycin is used, there is evidence that using a higher dose protocol leads to better efficacy in cases of MRSA infection [113]. In the 6 month to 4 year old age group, ceftriaxone should be added to clindamycin for empiric treatment when K kingae infection is suspected [31, 36, 101, 114, 115]. When a patient appears to have developed sepsis (ICU, fever, bacteremia, multi-focal disease, and/or pulmonary involvement) empiric treatment should include high-dose vancomycin and ceftriaxone.
Surgery is indicated for children with AHO who demonstrate failure to respond to antibiotic therapy, evolving sepsis, or who have imaging findings consistent with abscess formation (intra-osseus, sub-periosteal, or extra-periosteal) (Fig. 42.2). Surgical treatment involves irrigation and debridement of infected or necrotic bone. This is done by making a cortical window in the region of affected bone with care taken to avoid injury to the growth plate or peri-chondral physeal ring. The bone window should be large enough to perform an adequate debridement but not compromise the overall architecture of the bone. Surgical decompression reduces intra-osseus pressure, which may restore perfusion to the affected area and enhance anti-biotic delivery. One study demonstrated antegrade drilling of the femoral neck in children with AHO of the proximal femur to decrease the rate of contiguous septic arthritis and osteonecrosis [116]. Similar to septic arthritis, drains should be left in place to allow for evacuation of the space in the post-operative period. Pre-operative imaging must be studied carefully to identify all foci of infection. Any adjacent or contiguous abscesses (both sub-periosteal or extra-periosteal) should be drained at the time of bone decompression. Involvement of adjacent joints may occur in as many as 42 % of children with AHO [117]. If there is an effusion present in an adjacent joint, this should be aspirated at a minimum and evaluated for the presence of bacterial arthritis and/or irrigated and debrided when appropriate. Post-operative limitations on weight bearing and activity should be considered, depending on the location of infection and extent of debridement, to avoid pathologic fracture.
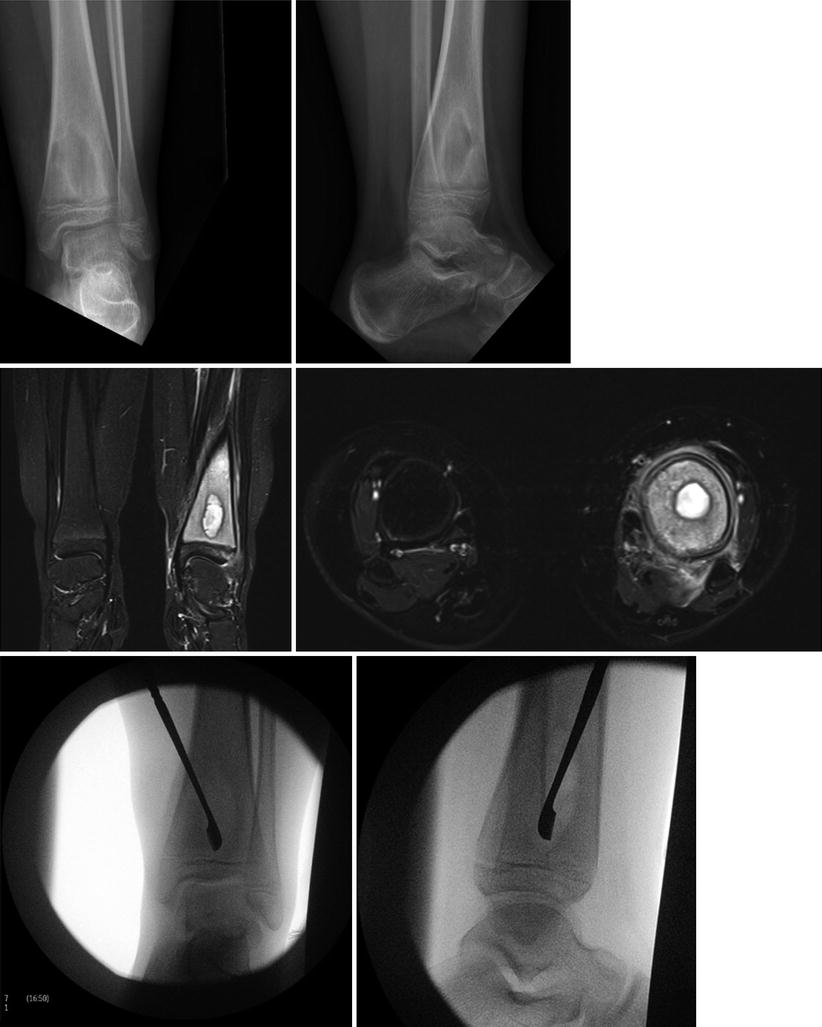

Fig. 42.2
Brodie’s abscess treated with incision and drainage
CRP and ESR levels should be monitored during the course of treatment of AHO to ensure appropriate response to treatment, including both antibiotics and surgery. The acute phase of AHO is accompanied by a rapid rise in CRP which declines at a moderate pace when the infection is being adequately treated. The ESR rises and declines more gradually, and may continue to rise even after treatment of the infection has been initiated [82, 118–124]. Monitoring of inflammatory markers should be accompanied by careful physical exam. Children who fail to demonstrate appropriate clinical or laboratory improvement after treatment has been initiated should be carefully evaluated to determine the need for repeat imaging with MRI. Repeat imaging in the early post-operative setting is advised only if the clinician feels there is a previously unaddressed focus (either local or remote) of infection. Otherwise, clinical and laboratory examinations should be used to guide the decision for repeat surgical intervention of known, or previously addressed foci [125–127]. In a series of 59 children with AHO, 104 repeat MRI studies were assessed regarding the indications for the imaging study and the impact on treatment. Twenty-eight of the MRIs were obtained within 2 weeks of the index imaging study because of a worsening clinical course. In this group of children a change in treatment occurred after 8 of the MRIs (29 %) compared with management changes in only 3 of the 76 MRIs (3.9 %) performed >14 days after the index study. Of the 11 children in whom repeat MRI changed the ultimate treatment plan, CRP levels were increasing in 7 and were elevated or unchanged in 4 [126]. Based on this is it not recommended that MRI be used in the routine monitoring of infection resolution as MRI in the aftermath of infection and surgical intervention has a prolonged appearance that is difficult to interpret. One study of 57 children with AHO showed that children with sustained elevation of CRP after 96 h after their initial surgery and who remain febrile while on antibiotic treatment have an increased likelihood of repeat surgical intervention and should be evaluated carefully for additional surgical treatment [128].
The convalescent phase of infection is accompanied by rapid decline and normalization of CRP (1–2 weeks) and more gradual decline of ESR (3–6 weeks). The patient can be considered for discharge and outpatient management with transition from parental to oral antibiotic therapy when there is sufficient clinical and laboratory improvement to ensure that the acute phase of the infection is over. In a study of 194 children with acute bacterial osteoarticular infections, a CRP of 3 mg/dL (in addition to improving clinical exam) was used as a guide for transitioning from parental to oral antibiotic therapy with 99.5 % success rate. Long term outcomes were similar to those expected with the more traditional extended parental therapy course [82]. Another study employed a CRP of 2 mg/dL as part of the criteria for discharge of children with osteomyelitis within a clinical practice guideline. They reported a shorter length of stay and lower hospital readmission rate among children in whom these criteria was applied [125]. Other institutions consider transition to outpatient care and oral antibiotic therapy when the CRP has reached 50 % of its peak value in addition to improvement in the clinical exam. In one study, 92 % of children with MSKI experienced a 50 % decline in CRP over 4 days [129]. While the clinical exam will not have normalized, there should be significant improvement in the following clinical parameters: temperature trend, inspection/palpation of site of infection for erythema, tenderness, swelling, range of motion, surgical site drainage and general appearance/demeanor of the child. The decision to terminate antibiotic therapy altogether is based on the normalization of laboratory markers and is typically dependent upon the normalization of ESR. The clinical exam should normalize over 6–12 weeks, depending on the location and extent of infection. If clinical and laboratory normalization does not occur as anticipated within a 3–6 week time frame, the clinician should continue antibiotic therapy and re-assess laboratory markers every 1–2 weeks. Failure to improve clinical or to normalize markers of infection should be considered a treatment failure and a repeat MRI should be considered to look for residual focus of infection or involucrum that is driving the ongoing inflammatory process. If identified, the clinical should perform repeat irrigation and debridement with new culture acquisition. Complications that can occur from osteomyelitis include: pathologic fracture, growth disturbance leading to limb length difference or deformity, joint contracture, osteonecrosis, spinal degeneration with loss of disc space and arthritis [130–135].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







