We begin our discussion with the fundamental changes that occur at the cellular level in a wound of the diabetic foot. It is beyond the limits of this chapter and the capacity of the authors to discuss in detail the complex pathologic mechanisms involved in the diabetic foot. We will limit our discussion to areas of clinical interest where new technologies have been developed to address issues in the micro- and macroenvironment of the diabetic foot.
A basic understanding of the histology and biology of skin and surrounding structures is necessary in evaluating and treating a chronic diabetic wound (
Fig. 69.1). There are four important components of skin that should be considered: epidermis, dermis, hypodermis (subcutaneous adipose tissue), and underlying deep soft tissues. The epidermis is the most superficial layer of skin. The majority of the epidermis has no direct vascular supply and the superficial layers are sloughed continuously. Directly below the epidermis, the dermis is often regarded as the most important skin component to wound healing due to the fact that granulation tissue is usually seen at this level. The hypodermis contains adipose tissues where neurovascular bundles are located. Finally, the underlying tissues include fascia, tendon, and bone, all of which have potential for granulation tissue formation. Inflammatory cells and cells that make up the skin contain important growth factors. Specifically, one should note that fibroblasts and keratinocytes are of key importance in the process of tissue regeneration and repair. Keep in mind
the different layers of tissue and their architecture as described above when treating a chronic diabetic wound so that an appropriate plan can be identified and implemented effectively.
FUNDAMENTAL PRINCIPLES OF WOUND HEALING
A brief review of the normal wound healing cascade is needed to better understand the chronic, nonhealing wound. The classic model of wound healing has been described to involve three phases (
11). The first phase is inflammation that begins after the initial insult when platelets adhere to the site of injury and to each other to form a hemostatic plug. Individual platelets contain intracellular structures known as alpha-granules that contain growth factors (cytokines), clotting factors, and other proteins involved in wound healing. After activation by thrombin, platelets aggregate to the area of injury and release the contents of their alpha-granules to initiate clotting. The platelet aggregate (hemostatic plug) serves as a barrier to the external environment. Platelet-derived growth factor (PDGF) is one of the key components released by the alpha-granules. PDGF is also found in macrophages as well as endothelial cells (
12). PDGF is involved in the formation of connective tissue, promotion of revascularization, production of granulation tissue, epithelialization, wound contraction, and wound remodeling (
13,
14 and
15). Further, platelets release transforming growth factorbeta (TGF-β) and platelet-derived angiogenesis factor (PDAF), which play key roles in wound matrix production by promoting collagen production and new capillary formation. PDGF also acts to recruit and activate proinflammatory cells such as fibroblasts, macrophages, monocytes, and neutrophils. These cells, in turn, also secrete growth factors including TGF-β, fibroblast growth factor (FGF), endothelial growth factor (EGF), and vascular endothelial growth factor (VEGF). These cellular interactions and chemotactic communications are critical elements in the wound healing cascade.
The second phase of wound healing is epithelialization. Epidermal cells begin to proliferate and migrate to formulate linkages with each other and begin depositing basement membrane components and also reorganize and degrade the extracellular matrix. Growth factors are intricately involved in this phase as well. Neovascularization occurs at the wound bed, causing the formation of granulation tissue, which infiltrates the temporary matrix. Fibroblasts play a critical role in orchestrating the reorganization of the extracellular matrix into a collagenous matrix through the use of proteases and other enzymes. Growth factors, such as VEGF, contribute to the stimulation of angiogenesis to support wound healing.
The final phase of wound healing involves tissue remodeling. This involves wound contraction facilitated by fibroblasts that have converted to myofibroblasts stimulated by growth factors. Collagen is then continually remodeled through enzymatic degradation by matrix metalloproteinases (MMPs) until final collagen deposition and wound reepithelialization has occurred. The diabetic foot wound displays a vastly altered wound healing cascade from that which we have just described. In the following sections, we will discuss the aberrations in the wound healing cascade that are of particular note to the compromised diabetic lower extremity wound.
It is important to note that MMPs act at every phase of “normal” wound healing. In the chronic, nonhealing wound, MMPs play a significant inhibitory role due to a pathologic imbalance in the amount and proportion of MMPs in the wound base. MMPs are part of a larger class of enzymes known as proteases and are released by keratinocytes, endothelial cells, and inflammatory cells. The expression of MMPs is potentiated by inflammatory factors such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). These proteases play an important role in the wound healing process by degrading extracellular matrix proteins, stimulating cell proliferations and migration, and promoting angiogenesis. MMPs are subdivided into collagenases (MMP-1,-8), gelatinases (MMP-2,-9), and stromelysins (MMP-3,-10,-11). Elevated levels of MMPs are thought to have detrimental effects on wound healing. MMPs have been measured at higher levels in chronic wounds as compared with acute wounds, and further, increased concentrations of MMPs have been reported in diabetic wounds compared with nondiabetic wounds (
16,
17). One of the key regulators of MMPs is the tissue inhibitor of metalloproteinase (TIMPs), which is a glycoprotein produced by fibroblasts. Chronic wounds display decreased levels of TIMPs, suggesting less regulation of MMP levels (
18).
Growth factors play an important role in wound healing. Growth factors serve a multitude of functions including recruitment and promotion of inflammatory cells, stimulation of cellular proliferation and regulation, angiogenesis, and the overall stimulation of the wound healing process. Some of the more important growth factors, as previously mentioned, are TGF-β, FGF, EGF, and PDGF (
19). The levels of these growth factors have been reported to be decreased in chronic wounds as compared with acute wounds (
20,
21 and
22). Further, TGF-β appears to be an inhibitor of MMPs and promotes the synthesis of TIMPs (
23,
24). FGF and EGF play many roles in wound healing, but are particularly important for keratinocyte-induced epithelialization (
25,
26). PDGF plays a major role in the recruitment and regulation of inflammatory and immunologic cells such as fibroblasts and macrophages, as previously described.
VASCULAR DYSFUNCTION
Vascular compromise also plays a major role in the chronicity of wounds. It is important to remember that a diabetic wound is not always ischemic; however, due to the vascular disease associated with diabetes itself, wounds may progress in this
direction. Vasculopathy associated with diabetes has two major components: macrovascular and microvascular processes. Macrovascular pathologic processes include cerebrovascular, cardiovascular, and, more pertinent to our discussion, peripheral vascular/arterial disease (PVD, PAD). Microvascular dysfunction revolves around issues with the microcirculation at the level of arterioles and capillaries and is more of a dysfunction in physiology rather than anatomy. Again, a detailed discussion of this complicated topic is out of the scope of this chapter; however, fundamental issues associated with the vascular process will be addressed.
PAD is one of the devastating sequelae of diabetes (
27). Diabetic patients with PAD have a higher risk of lower extremity amputations (
28). The hallmark of the atherosclerotic process is fibrofatty plaque deposits initiated by an inflammatory process within the vessel walls. The plaques may progress to a point at which complete occlusion may occur. Wound care specialists are often the first to identify systemic ischemic disease as manifested by the symptoms of a nonhealing wound. The ischemia discovered will often persist much further systemically and in fact is a common marker for carotid and cardiac vessel disease. “Critical limb ischemia” is a term used to describe the point at which, without intervention, limb amputation is a strong probability (
29). Revascularization can be conducted through open or, more commonly now, through endovascular techniques. Open techniques may include the harvesting of the greater saphenous vein to be used as the vessel that bypasses the area of blockage. Endovascular techniques utilize a variety of ablative technologies to remove focal plaques through minimally invasive techniques (
30). The analogy of a plumber laying down new pipes around a clog (open) versus the use of a “rotorooter” to remove the clog within the clogged pipe (endovascular) is appropriate. Prior to lower extremity revascularization, an arteriogram or a magnetic resonance angiography (MRI) is performed to identify the areas of occlusion.
The microvascular environment (namely arterioles and capillaries) is also altered in the patient with diabetes (
31,
32 and
33). Generally, there appears to be two components in the diabetic microvascular system that are altered: first, there is an increase in vascular permeability, and second, there is an impairment in the regulation of vascular tone and local blood flow (
34). This, in part, has to do with a thickening of the capillary basement membrane and a decrease in capillary size (
35,
36). There are numerous factors involved in the pathologic microvascular environment in the diabetic foot, so we will limit our discussion to those associated with currently available clinical interventions.
Endothelial function, or more accurately, dysfunction, has significant effects in the microvasculature. Specifically, the role of nitric oxide (NO) in regulating vascular tone in the diabetic patient has been a major focus of research. NO is an endogenous gas produced by cells with many diverse physiologic effects. The substrate arginine is converted by the enzyme nitric oxide synthase (NOS) to citrulline with the liberation of NO. After release, NO has a half-life of seconds with subsequent binding to receptors on or within the cell causing a second messenger cascade. The result of this cascade depends on the type of NOS. There are many subtypes of NOS, including nNOS, which is found in neurons; iNOS, which is an inducible form found throughout the body; and eNOS, which is found in vascular endothelial cells. Of particular interest are iNOS and eNOS, which have implications in the lower extremity and wound healing cascade.
Endogenous NO acts upon endothelial cells causing dilatation of vessels (both arterial and venous). Contrarily, inhibition of NO synthesis causes vasoconstriction and hence hypertension (
37). Evidence also suggests that there is decreased NO activity with an increase in activity levels by vasoconstrictors in the diabetic rat model (
38). Further, the vessels of insulin-dependent patients with diabetes demonstrate less responsiveness to NO and potentially a decrease in availability of NO (
39,
40 and
41). NO has also been shown to increase blood flow to the microcirculation adjacent to wound sites (
41).
There have been a variety of clinical strategies employed in an attempt to enhance or augment NO-related endothelial function. One strategy involves the use of folic acid, vitamin B
6, and vitamin B
12. Supplementation with folic acid has been shown to improve NO-mediated endothelial function in diabetic patients (
42,
43). Folate has also been used in combination with B
6 and B
12 in an attempt to decrease homocysteine levels (
44,
45 and
46). Elevated homocysteine levels have been implicated in endothelial dysfunction (
47,
48,
49 and
50). Another strategy involves the use of NO liberators including topical applications of nitroglycerin and oral formulations of L-Arginine. Both have been reported to promote wound healing (
51,
52,
53,
54,
55,
56,
57,
58 and
59). However, others have suggested that NO augmentation may not be beneficial or effective in a chronic wound environment (
60,
61,
62,
63 and
64).
NO also has more direct implications on the wound environment itself. NO has been measured at higher levels in proximity to wound sites (
60). NO appears to be maximally expressed early in the wound healing process, with sustained decreased levels for the first couple weeks after injury (
65). Cytokines stimulate macrophages and fibroblasts to produce NO (
66 and
67). NO appears to have cytotoxic properties, which suggests some level of antimicrobial activity (
68). Further, inhibition of NO synthesis retards collagen synthesis and deposition, which we know are key components in providing principal strength characteristics of wounds (
69).
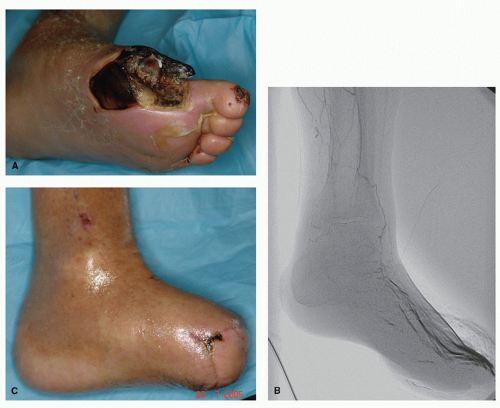
Proper assessment and treatment of vascular dysfunction must be addressed prior to or in conjunction with wound care modalities (
Fig. 69.2). If blood flow is compromised to the affected extremity, the wound will not heal or will be severely retarded in healing. The wound is entirely dependent on adequate nutrient and oxygen delivery. Hence, vascular evaluation and possibly consultation is necessary in the treatment of a chronic diabetic wound.
PERIPHERAL NEUROPATHY
Peripheral neuropathy has been cited as a pivotal process that contributes to the chronicity of diabetic wounds and can affect up to 66% of patients with diabetes (
70,
71,
72 and
73). Peripheral neuropathy can be generally defined as a progressive loss of peripheral nerve fibers. In the diabetic population, this typically presents in a symmetrical fashion and involves sensory, motor, and autonomic neuropathy. The etiology of peripheral neuropathy is most attributable to hyperglycemia (
74). Hyperglycemia contributes to metabolic disturbances that negatively impact nerve function. Other contributing factors include focal areas of nerve entrapment, which can lead to degeneration of the nerve fiber. Nerve entrapment may benefit from nerve surgical decompression (
75).
Sensory neuropathy can often present as a painful process, with patients reporting symptoms of “pins and needles” or
“burning” sensation in its early stages. Peripheral neuropathy may progress to a point in which complete loss of sensation occurs. The typical insensate process begins distally at the toes and advances proximally and is said to be in a “stocking and glove” distribution. The critical aspect of peripheral neuropathy is that patients are unaware of focal areas of trauma, which may lead to the development of a wound (
72). This wound continues to be traumatized and inadequately treated and hence becomes chronic in nature.
Motor and autonomic neuropathies also contribute to the chronicity of a wound. Motor neuropathy may lead to an imbalance of muscles or muscle atrophy in the lower extremity. This may lead to areas of increased pressure that may subsequently lead to tissue breakdown. Autonomic neuropathy involves the denervation of the sympathetic nervous system, leading to arterial blood flow being shunted away from the nutrient capillaries. This arterial to venous shunting diverts the nutrients and oxygen away from the underlying soft tissue structures of the plantar aspect of the foot. Hence, tissues become less tolerant to stresses leading to tissue breakdown. This process may also explain loss of sweat production and fat pad atrophy.
NO also plays a role in autonomic neuropathy. Human studies have demonstrated decreased NO production in diabetic patients with peripheral neuropathy (
76). Associated with this fact, homocysteine levels are elevated in persons with type 2 diabetes and peripheral neuropathy (
77). Peripheral neuropathy is associated with a decrease in perfusion to peripheral nerves, causing hypoxia to the nerves (
78,
79). Several different clinical strategies have been developed to offset the endothelial dysfunction associated with peripheral neuropathy. Technologies utilizing monochromatic nearinfrared photoenergy therapy have been postulated to induce the release of NO through photoenergy, thereby increasing blood flow to the peripheral nerves (
80,
81,
82 and
83). Topical application of NO in the form of a spray has also been used for the treatment of painful peripheral neuropathy with good success (
84). Further, oral formulations utilizing the combination of supplements (folate, B
6, B
12), as described above, have been used to manage peripheral neuropathy (
42,
43,
44,
45 and
46).
Other oral medications targeting symptomatic management of peripheral neuropathy that is potentially unrelated to NO have been utilized including tricyclics, selective serotonin reuptake inhibitors, anticonvulsants, antiarrhythmics, narcotics, and nonsteroidal anti-inflammatories (
85).
Peripheral neuropathy is a significant contributing factor to the development and chronicity of a diabetic wound (
86). Proper management of peripheral neuropathy with early intervention utilizing some of the treatment options discussed above may prevent the onset of a diabetic wound and also retard the progression of neuropathy that may lead to an insensate foot (
Fig. 69.3).