Fig. 4.1
Cranial suture, view from the top
4.1 Mechanisms of Cranial Deformities
Skull growth after the premature fusion of a single suture was first described by Virchow in 1851. He observed that growth was restricted in a plane perpendicular to a fused suture [3]. This theory, however, failed to explain the compensatory growth patterns that resulted from the fused suture. Further analysis [4] revealed that skull growth patterns after suture fusion could be predicted using the following rules: (a) cranial vault bones that are prematurely fused act as a single bone plate with decreased growth potential; (b) asymmetrical bone deposition occurs mainly at perimeter sutures, with increased bone deposition directed away from the bone plate; (c) sutures adjacent to the stenotic suture compensate in growth more than those sutures not contiguous with the closed suture; and (d) enhanced bone deposition occurs along both sides of a no perimeter suture that is a continuation of the prematurely closed suture.
Thus, premature lambdoid fusion results in limited growth in the ipsilateral anteroposterior plane and in a compensatory growth of sutures adjacent to the fusion, including the sagittal and contralateral lambdoid sutures. This growth compensation results in the trapezoid formation of the head when evaluated from a vertex view. In comparison, deformational plagiocephaly is simply the adaptation of the skull bones to an external force, resulting in displacement of the bones to counteract the external force. Compression of the ipsilateral occipital region results in flattening and ipsilateral frontal bossing. This force translation causes the skull bones to shift along the axis of the force applied, thus creating a parallelogram formation of the head when evaluated from a vertex view [5].
While a complete understanding of the etiology of craniosynostosis is unavailable, current theories implicate both environmental and genetic influences.
The heterogeneous nature of craniosynostosis is reflected, in part, by the diverse environmental factors that have been reported to be associated with its development. A small sample of these environmental factors includes maternal smoking, the maternal habit of living at high altitude, and certain paternal occupations, such as agricultural and mechanic work. Most of these associations have not been confirmed with large-scale studies, and the mechanism by which these environmental factors predispose the fetus to develop craniosynostosis is unknown. The intrauterine environment also has been implicated in the development of craniosynostosis. The current thinking is that mechanical stress on the developing cranium due to intrauterine head constraint is causative in at least some cases.
At a cellular level, the neural crest-derived precursor cells of the cranial bones form discrete condensations by the middle of the first trimester. As these condensations expand and ossify, sutures form at the leading edges of adjacent calvarial bones. A paradigmatic shift in our understanding of the pathogenesis of PPP emerged as it became appreciated that diffusible factors from the underlying dura are necessary for this initial formation and maintenance of sutures. Expansion of the cerebral hemispheres likewise is essential to the maintenance of suture patency.
Thus, microcephaly predictably leads to early suture closure, whereas hydrocephalus prolongs suture patency. Studies have shown that sutures are sites of bony adaptation that normally allow for cranial expansion, compliance, and adaptability well into the third decade of life. Premature suture fusion, generally occurring before birth, severely disrupts this developmental program.
Positional plagiocephaly (PP) is an abnormal head shape that lasts beyond 6 weeks after birth. This is caused by prolonged external pressure to the skull by lying on one area of the head and often becomes a flattened area. This may also be due to positioning in utero, premature birth, birth trauma, or torticollis. When severe, this can lead to abnormalities in facial bones as well.
Although the head may be asymmetrical immediately after birth, deformities are usually absent at birth and are highlighted in the first 2–4 months of life [6], and in particular to 7 weeks [7].
The utmost severity occurs at about 4 months. Since the baby is put to sleep on its back after birth, even in the absence of risk factors, its head, resting repeatedly on the same area, quickly runs to flattening and, lacking any preventive intervention, the deformity is likely to persist if not worsen.
4.2 Pathogenetic Mechanisms of PPP
PPP is produced by pressure from the outside on part of the skull. It can occur while the baby is still developing in the womb, but in recent years, flattening occurring after the baby is born has become much more frequent. PPP develops when an infant sleeps or rests on one part of the head most of the time. It has become more common because parents are now advised to place infants on their backs for sleep to help prevent SIDS. Extended use of car seat carriers, swings, and bouncers also place infants on their backs for long periods of time and may change head shape.
It has long been known that the skull of the newborn increases passively in response to the minimum internal pressure due to the growth of the brain [8]. The flattening occurs when the growth of the skull meets resistances from external forces in its specific areas [9].
If the baby is put to sleep on a hard surface, it creates a contact between its head and the bearing surface: the force applied by the head on the surface is equal to the weight of the child’s head multiplied by the gravity force.
Newton’s first law states that there is an equal and opposite force acting from the surface to the baby’s head which resists the growth in the contact zone. In consequence of this, the volumetric growth occurs in areas where there is no resistance. This compensatory mechanism is then the cause of the flattening of the head [10]. The same mechanism is observed in growing pumpkins resting on the ground (Fig. 4.2).
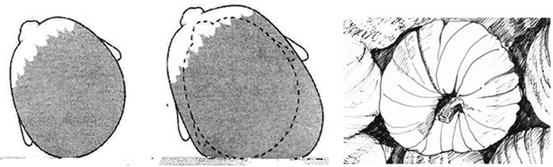

Fig. 4.2
Pathogenetic mechanism of deformation of the skull: Newton’s first law justifies the flattening of the occiput, similarly to the growth of a pumpkin on the ground (see explanation in the text)
This would explain why parents usually do not notice any flattening of the head in the first 6–8 weeks of life of the newborn [7, 11–17].
Still according to Newton’s first law, flattening is greater in males who have larger head and a more rapid rate of growth [2, 7, 11, 13, 15–20]. The risk increases in the case of twins, with the finding of cranial asymmetry in 56 % of cases [14, 21].
Although pressure on the occiput of the growing infant’s skull is the main cause of PPP, the origin for the side preference remains unclear. Multiple factors have been associated, encompassing intrauterine, neonatal, and environmental forces.
Decreased motility of the head after birth, which determines the preferred posture of the infant in supine position, is considered crucial in the mechanism of the onset of PPP. Some authors believe that muscle factors that limit the degree of mobility of the head and neck are often underestimated. Altered posture of the head at birth, such as postural torticollis (PT), may be present in 70 % of infants with plagiocephaly [6, 18, 22].
4.2.1 Torticollis Associated with Positional Plagiocephaly
The three most common causes of infantile torticollis are idiopathic muscular causes such as sternocleidomastoid fibrosis, structural anomalies in the cervical vertebrae, and neurologic or ocular causes, such as certain types of strabismus.
If the infant is born with congenital muscular torticollis, the turn and tilt of the head from the shortened sternocleidomastoid muscle initiates the side preference and the occiput flattens correspondingly.
Recently, several units have reported increasing numbers of babies presenting with head tilt and a reduced range of cervical motion, a form of torticollis apparently associated with PPP and unrelated to the etiologies enumerated above. A retrospective review showed a persistent escalation in referrals for both PPP and associated torticollis (not attributable to one of the three causes mentioned) over the study period. Anatomic and clinical features of PPP-associated torticollis were characterized and contrasted with the classic forms of torticollis. Torticollis can predispose to PPP, but in a large proportion of cases, torticollis appears to develop secondary to plagiocephaly [23].
Torticollis and PPP are closely related to each other and influence each other’s outcome. For example, when a baby prefers to look to the right (left torticollis), a right-sided flattening (plagiocephaly) could develop due to the infant’s soft skull. The longer the baby stays on this preferred side, the worse the flattening could get, making it more difficult to look the other way.
If the infant stays in this position for an extended period of time, the sternocleidomastoid muscle (SMC) may react to the preferred position and become short or tight. Outcomes for torticollis and PPP can be exacerbated without treatment. For instance, untreated PPP can predispose one SCM to become weaker and the other SCM to become tighter. The baby will therefore stay on the affected flattened side continuing the cycle of head flattening and muscle weakening.
A typical presentation of muscular torticollis includes lateral flexion of the neck to one side and rotation to the opposite side caused by shortening of the SCM. Torticollis may be congenital or acquired, due to intrauterine positioning, ocular muscle imbalance, or positional plagiocephaly. Once considered by some to be merely cosmetic, torticollis is now known to coincide with abnormal tone and motor delays. Early physical therapy has been shown to improve torticollis outcomes and decrease the duration of therapy, especially when the therapy is started prior to 2 months of age.
4.3 Risk Factors for PP
Several conditions can increase the chance that a baby will develop PPP, including maternal, perinatal (during pregnancy and at delivery), and postnatal factors. Several prospective cohort studies of infants [6, 7] and case series studies of infants with PPP [24, 25] have suggested that possible risk factors for PPP include assisted delivery, firstborn child, male sex, cumulative exposure to the supine position, and infant neck problems. PPP is also associated with intrauterine constraint [26]. Although many of these risk factors are not preventable, supine sleep position seems to be the best independent predictor of PPP of these factors [27]. Infant care trends over time and cultural norms seem to support this finding, as evidenced by a recent shift in recommendations for infant positioning in the United States.
Among maternal risk factors, intrauterine and extrauterine factors such as uterine crowding due to a large fetus, multiple fetuses, oligohydramnios, small maternal pelvis, and/or prominent lumbar spine are to be considered. Even the left occiput anterior (LOA) position in utero predisposes the fetal head to unilateral pressure. Maternal age over 35 years, ethnicity, and schooling are also to be taken into account.
Perinatal factors include male gender [7], breech or cross-presentation, any birth trauma (pressure on the head by a tight birth canal, forceps delivery, and prolonged labor) [12], macrocephaly, hydrocephalus, and all causes of hypomobility of the fetus, leading to skull compression: oligohydramnios, premature rupture of membranes, infants born prematurely or with low birth weight, firstborn infant, multiple pregnancy, decreased fetus’ spontaneous motility, hypotonic infants presenting with weak neck muscles, congenital hip and spine problems, and torticollis [28].
Once born, all babies have some pressure on their skulls from such things as mattresses and baby carriers. However, some infants show a preference for sleeping or sitting with their heads turned consistently in the same position, which may lead to PPP.
4.3.1 Maternal Risk Factors
Some authors have postulated that the occipital flattening develops in utero. Most fetuses lie in the left occiput anterior position. The pressure of the maternal pelvic bones on the fetal head could give way to the unilateral flattening. Infants from multiple births (twins, triplets, and so on) with in utero crowding are at an increased risk for PPP. In one study examining newborns 24–72 h after birth, the incidence of localized cranial flattening in singleton infants was 13 %, whereas in twins, the incidence was 56 %. Oligohydramnios similarly could cause intrauterine crowding and pressure on the developing head.
The restricted space inside the mother’s womb can create excessive contact in certain areas of the baby’s head. This is often the cause of deformation in babies positioned in a breech position (the baby’s head becomes wedged under the mother’s ribs), cramped in utero because of multiple fetuses, or in babies who spend excessive time with the head confined in the birth canal. In utero constraint can be caused by a variety of factors including a multiple pregnancy with two or more fetuses (during twins or multiple pregnancy the babies may be too crowded to move about freely in the womb, therefore receiving constant pressure on their skulls), a small or unusually shaped uterus, uterine fibroids, a small maternal pelvis, or an inappropriate amount of amniotic fluid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







