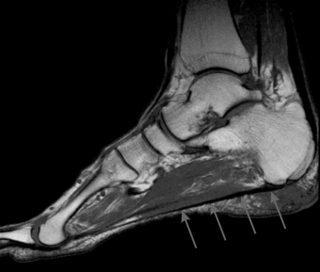
5.5 Diabetic foot Diabetes mellitus is a chronic disease affecting over 200 million people worldwide, a number that will double in the next 20 years (www.diabetesatlas.org/content/diabetes). Diabetes may lead to several vascular and neurological complications, including ulceration, infection, or destruction of deep tissue in the foot. This “diabetic foot” affects approximately 15% of patients (Boulton et al. 2004). The foot ulcer is the key clinical problem in the diabetic foot that can cause infection and lead to lower-extremity amputation. Foot ulcer incidence is 7.2% per year in diabetic patients with peripheral neuropathy (Abbott et al. 1998). Peripheral neuropathy leads to a loss of protective sensation, which makes the patient unaware of foot trauma caused by the repetitive action of elevated mechanical foot pressures. These high foot pressures are secondary to structural abnormalities which include claw toe and Charcot deformity, prominent metatarsal heads, and changes in subcutaneous and periarticular connective tissue (i.e., tendon, fascia, ligaments, and joint capsule). Morphological changes in the plantar fascia and Achilles tendon, and limitations in joint mobility have been reported in diabetes. Changes in these foot structures share a common etiology related to long-standing hyperglycemia and they may influence foot mechanics during gait and lead to foot ulcers. The goal of this chapter is to provide insight into the changes that occur as a result of diabetes in the plantar fascia, Achilles tendon, and joint mobility of the lower extremity. The underlying mechanisms of these changes will be discussed, together with their biomechanical and clinical implications, as well as available treatment options. Different methods can be used to assess changes in foot structure, joint mobility, and biomechanical function in the diabetic foot. Morphological changes in subcutaneous and peri-articular tissues in the foot and lower leg are best assessed using in-vivo imaging techniques. With high resolution ultrasonography the geometric boundaries of superficial structures in the foot and lower leg can be assessed, from which tissue thickness can be measured (D’Ambrogi et al. 2003; Giacomozzi et al. 2005). More detailed qualitative and quantitative information of superficial and deep structures can be obtained using magnetic resonance imaging (MRI). MRI is superior to other imaging techniques in distinguishing soft tissue such as muscle, tendon, ligament, fascia, and fat, and can be used to measure tissue thickness and assess the presence of rupture. The plantar fascia can be tested functionally using Jack’s test, in which the hallux is passively dorsiflexed while weight-bearing (Chuter & Payne 2001). A normal response is tightening of the fascia and a raise of the foot arch. Failure may indicate fascia dysfunction or rupture. The so called “prayer sign” was originally used to classify patients with limited joint mobility (LJM). This is present when the patient fails to approximate the metacarpal–phalangeal joints while opposing the palmar surfaces of the hands in a praying position. Although this method is simple in use, it is not a direct measure of LJM in the foot. Typically, a goniometer is used to assess mobility of the foot and ankle joints in a nonweightbearing position. Goniometric measurements of joint mobility are quite reliable, with reported coefficients of variation of 8.5% for the subtalar joint and 7.4% to 11.0% for the first metatarsal–phalangeal (MTP) joint (Delbridge et al. 1988; Zimny et al. 2004). Mobility and stiffness of the first MTP joint can be assessed using a mechanical testing device (Birke et al. 1995). Joint stiffness can be calculated from vertical displacement of the first metatarsal head plotted against the applied force to the metatarsal head. Biomechanical function of the foot is most often assessed in diabetic patients by measuring the dynamic pressure distribution underneath the foot. Patients walk barefoot across a platform, which consists of a matrix of hundreds to thousands of sensors measuring vertical pressure. Most often the peak pressure and the time integral of this peak pressure are calculated for multiple anatomical regions in the foot, so that conclusions on local pressure effects can be drawn. Structural changes in plantar fascia, Achilles tendon, and joint mobility in diabetic patients share a common etiology, namely nonenzymatic glycosylation of structural proteins in connective tissue secondary to the permanent hyperglycemic state in patients with diabetes mellitus (Bailey 1981; Brownlee et al. 1988). With nonenzymatic glycosylation, free glucose spontaneously attaches to structural proteins such as collagen and keratin (Schnider & Kohn 1980; Delbridge et al. 1985). Glycosylation of collagen causes an increase in intermolecular cross-linking and significant alterations in the structural stability of different collagen-rich subcutaneous and peri-articular connective tissues, such as tendon, ligament, fascia, and joint capsule (Delbridge et al. 1988). Glycosylation of keratin causes hyperkeratosis of the skin (Delbridge et al. 1985). These tissues show reduced flexibility, increased tensile strength, and other morphological adaptations (Crisp & Heathcote, 1984), which are likely the cause for some of the structural abnormalities found in the diabetic foot. The plantar fascia, or aponeurosis, is an important connective tissue structure which provides support, rigidity and stability in the foot under dynamic conditions (Hicks 1954; Sarrafian 1983; Sharkey et al. 1998). The aponeurosis consists of longitudinally oriented collagen and elastic fibers. It originates from the posteromedial calcaneal tuberosity and divides into five bands at the mid-metatarsal level, with each band inserting into the plantar plate and the skin. (Bojsen-Moller & Flagstad 1976; Theodorou et al. 2000, 2002). According to the windlass mechanism described by Hicks (1954), extension of the MTP joint during the propulsion phase of gait causes the aponeurosis to tighten and to draw the calcaneus and the metatarsal heads together. This results in a raised longitudinal arch and rearfoot supination, thereby making the foot a stable rigid lever in propulsion. On weight bearing in the first half of the stance phase, the arch flattens, which increases tension in the aponeurosis. This tension unwinds the “windlass”, causing flexion at the MTP joint. Failure of the plantar aponeurosis most often occurs proximally. In nondiabetic subjects, aponeurosis rupture may reduce its stabilizing action and lead to collapse of the longitudinal arch or claw toe deformity, and may increase pressures in the forefoot (Hicks 1954; Sarrafian 1983; Sharkey et al. 1999). Fascia thickening may alter the height of the longitudinal arch (Arangio et al. 1998). The effects that diabetes has on the plantar fascia are largely unknown. The plantar fascia of diabetic patients with claw toe deformity showed discontinuity, indicating rupture, in one MRI study (Taylor et al. 1998). The authors suggest that the effects of nonenzymatic glycosylation may render the aponeurosis less compliant and more prone to rupture. However, none of the patients with claw toe deformity in another MRI study showed discontinuity of the aponeurosis (Fig. 5.5.1) (Bus 2004). Also, signal intensity increases and substantial thickening of the aponeurosis at the calcaneal insertion compatible with plantar fasciitis have been found in neuropathic diabetic patients. However, these changes did not differentiate patients with toe deformity from those without (Bus 2004). Clearly, the data on fascia rupture and the role the aponeurosis plays in causing toe deformity in diabetic patients remain inconclusive. The plantar fascia may be thicker in diabetic patients than in healthy controls (D’Ambrogi et al. 2003; Bolton et al. 2005). High-resolution ultrasound images showed on average 2.0 mm thickness at the calcaneal insertion in healthy controls, 2.9 mm in diabetic patients, 3.0 mm in neuropathic diabetic patients, and 3.1 mm in diabetic patients with a history of foot ulceration (D’Ambrogi et al. 2003). With computed tomography, a thicker aponeurosis was found in diabetic patients than in healthy controls (mean 4.2 vs 3.6 mm) (Bolton et al. 2005). Increased fascia thickness in diabetes is likely also associated with nonenzymatic glycosylation of collagen; a reduction of 30% in collagen content in the plantar fascia has been found in diabetic patients (Andreassen et al. 1981). Apparently, with long-standing diabetes, geometric changes of the plantar fascia shown as thickening of this connective tissue structure may occur. Thickening of plantar fascia can influence biomechanical foot function in diabetes. Dynamic forefoot pressures were found to be significantly higher in diabetic patients with thicker plantar fascia than in diabetic and healthy control subjects with thinner plantar fascia (D’Ambrogi et al. 2003; Giacomozzi et al. 2005). Additionally, a significant correlation (r = 0.52) was found between fascia thickness and forefoot pressure in these patients. This association may be explained by the role plantar fascia plays in countering the flattening of the foot during mid-stance of gait. The vertical forces acting on the forefoot during arch flattening are countered by horizontal forces generated in passive structures such as the plantar fascia, which try to tie the forefoot and rearfoot together. With fascia thickening, resistance increases, meaning that larger vertical forces measured as higher pressures are required at the forefoot in order to flatten the foot during stance. In patients with Charcot’s neuroarthropathy, plantar fascia dysfunction or rupture may be indicated, based on a negative response to Jack’s test (Chuter & Payne 2001). Additionally, rupture of the plantar fascia has been suggested as a potential factor in the increased forefoot-to-rearfoot pressure ratio that is found in neuropathic diabetic patients when compared to healthy controls (Caselli et al. 2002). A suggested forefoot drop as a result of fascia rupture (Sharkey et al. 1999), may cause increased loading in the forefoot and explain these results. More research is required to improve our understanding of the role that the plantar fascia plays in altering biomechanical function of the foot in diabetes. The clinical implications of structural changes in the plantar fascia in diabetic patients are not known. This may be the reason for the paucity of data on treatment options. Alterations in foot function associated with plantar fascia dysfunction may be indicative of its contributing role in foot ulceration in diabetic patients (Caselli et al. 2002; D’Ambrogi et al. 2003). However, direct assessments of this relationship have not been made to date. The Achilles tendon is a fibrous structure which originates at the midcalf position and inserts into the middle part of the posterior surface of the calcaneus. It is the thickest and strongest tendon in the body. Contraction of the calf muscles pulls the Achilles tendon, resulting in plantar flexion at the talocrural joint. Injuries or abnormalities of the Achilles tendon include tendinosis, rupture, and shortening (equinus deformity). In diabetes, the Achilles tendon has been studied mainly for morphological adaptations (length and thickness) and for lengthening of the tendon as treatment option in foot ulcer patients.
Introduction
Methodology of testing
Assessment of fascia, tendon, and ligament
Assessment of joint mobility and stiffness
Pressure distribution measurement
Nonenzymatic glycosylation
Plantar fascia
Rupture and fasciitis
Plantar fascia thickening
Biomechanical implications
Clinical implications and treatment
Achilles tendon
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine