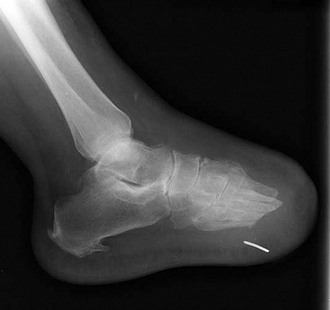
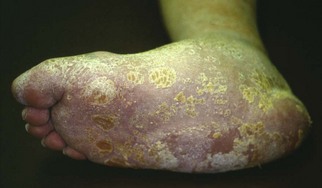
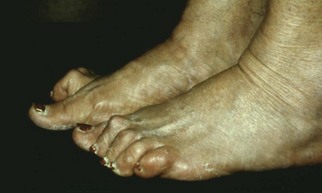
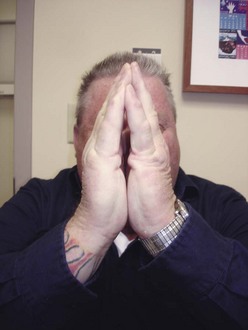
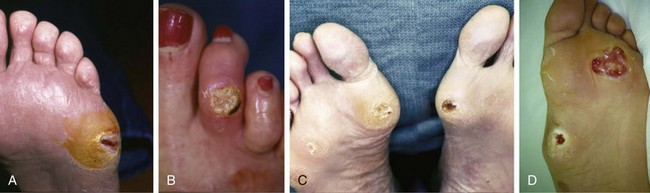
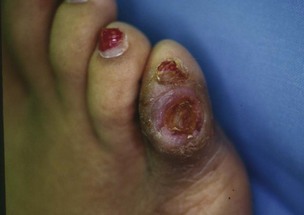
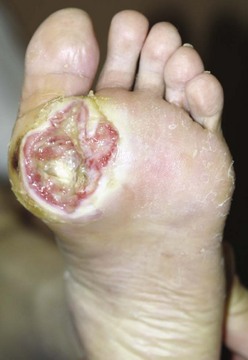
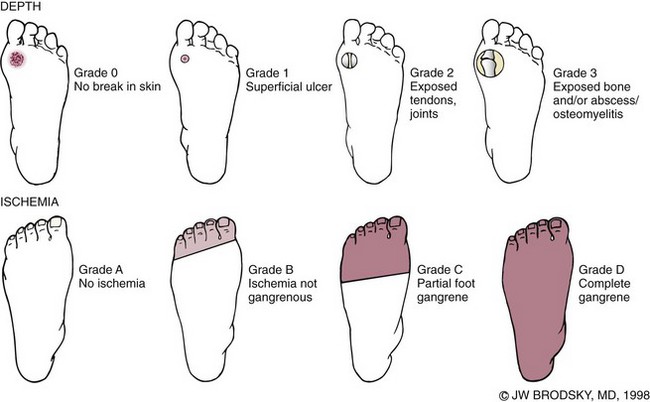
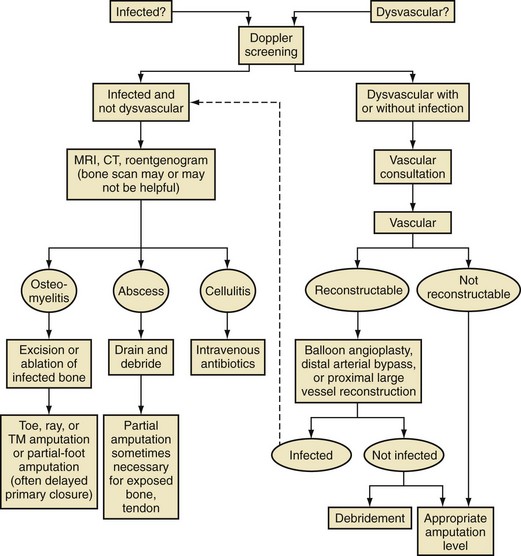
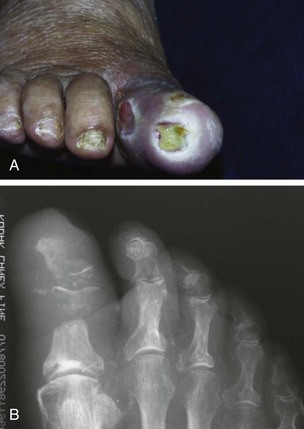
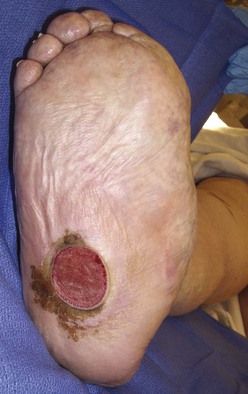
Chapter 27 COMMON CLINICAL PROBLEMS ASSOCIATED WITH DIABETES Wound Closure and Soft Tissue Reconstruction Treatment of Charcot Arthropathy Unique Considerations in the Treatment of Ankle Neuropathic Arthropathy Surgical Decision Making for Charcot Ankle Charcot Ankle Arthropathy Associated with an Open Wound Unstable Ankle Deformity Patterns Ankle Fractures in the Diabetic Patient Postoperative Management: Prolong Length of Immobilization Diabetes mellitus was described in the medical writings of fifth century BC Greece, and the name itself is from the Greek (dia, through; bainein, to go; melitos, honey). It was diagnosed by the sweet taste of patients’ urine, their excessive thirst, and the agony of their inevitable death.273 Although the method of diagnosis was slightly less crude by the beginning of the 20th century, little else changed in the natural history or course of this disease for 2500 years. Only with the discovery of insulin by Banting and Best in 1922 did the modern era of diabetes as a nonfatal chronic disease begin. Since that landmark discovery, medicine has unfolded the myriad multisystem complications by which this disease is now well known. The American Diabetes Association currently estimates the population of diabetic patients in the United States is more than 25 million and rising as the average age in the United States increases, because the incidence of diabetes increases with age.96 The Centers for Disease Control and Prevention estimates that over a quarter of these are undiagnosed cases.96 Foot problems, especially infections, are the most common disorder, necessitating hospital admission. More than 60% of nontraumatic lower limb amputations occur in patients with diabetes.96 The incidence of diabetes has increased dramatically in people older than 40 years, and an estimated 26% of the population older than age 65 is affected. The cost in morbidity, mortality, and time lost from work and family is enormous. The economic impact of diabetic foot problems and their sequelae on the health care system is mammoth.15,139,298 A review of diabetic patients in a medical clinic showed a 68% incidence of some form of foot disease. Most problems were mild, with very early changes, such as callus formation (51%) and hammer toes (32%); some were more apparent as sources of eventual injury and disease, such as sensory (34%) and autonomic (25%) neuropathy; however, all represented the early requisites that can lead to later problems of ulceration and infection.190 Diabetes is a metabolic disease resulting from inadequate insulin secretion, decreased responsiveness to insulin, or both. The hyperglycemic state experienced in diabetes is considered multifactorial, but most specialists agree that the long-term control of blood glucose levels reduces a patient’s risk for the known long-term complications of diabetes, such as vascular disease, nephropathy, retinopathy, and neuropathy.198 Careful control of blood glucose levels is critical for prevention as well as treatment of diabetic foot pathology. All diabetic patients should be followed closely by a primary care physician or endocrinologist experienced in the treatment of diabetes. Neuropathy, especially sensory neuropathy, is the preeminent source or initiating event of almost all ulcerations and most infections (Fig. 27-1). Peripheral vascular disease often coexists with neuropathy and contributes to poor or delayed healing, but it is seldom the initiating event. Vascular insufficiency causes tissue ischemia and can produce gangrene, prevent healing because of marginal nutrition of tissue, or even cause ischemic pain. However, the loss of protective sensation combined with acute trauma, recurrent trauma, repetitive trauma, or even microtrauma leads to most instances of soft tissue breakdown in the diabetic foot.35,61,125,238 The exact cause of diabetic neuropathy remains to be fully elucidated; however, autopsy studies have indicated that changes in the vasa nervorum, with resulting ischemia to the nerves, are the major pathway. The two theories regarding these microvascular changes in the nerves postulate the accumulation of sorbitol and intraneural accretion of advanced products of glycosylation. The only current possible treatment involves enzymatic blocking of the biochemical path toward sorbitol, but the efficacy of this treatment remains unproved.29 For all practical purposes at this time, neuropathy remains an irreversible condition that tends to progress gradually, and it lies at the heart of most foot disease in the diabetic patient.379 Risk of developing neuropathy is associated with poorer glucose control, age, and height.6,298 Brand and Ebner wrote a pioneering paper in 1969 describing the importance of pressure in the development of insensitive hands and feet. This study called for objective methodology to quantify pressure and heralded the development of this area of investigation.64 Research using optically based52,53,57,135,149 and electronically based92,155 pressure measurement systems have demonstrated abnormal, increased pressures under the diabetic foot that have been postulated for so long but could not be measured or quantified before these developments.18 One early study57 used the optical pedobarograph to examine patients with neuropathy for abnormal plantar pressures, defined as pressures greater than 10 kg/cm2. The same patients, when examined 3 years later, showed that some of these pressure areas resolved, whereas new ones appeared in others; 5 of 6 patients in the group of 39 who developed ulcers developed them in the areas identified previously as locations for abnormal pressure. In a study by the same group,83 14 of 44 patients thought clinically not to have neuropathy were discovered to have abnormal plantar pressures.57 When tested for vibratory sensation and sensory nerve conduction, these patients showed significantly decreased function, which suggests that the pathologic pressures that lead to ulceration can occur in conjunction with neuropathy but at a point before the neuropathy itself is apparent. A study comparing diabetic patients who have a history of ulcerations with diabetic controls without such a history revealed that both the severity of neuropathy and the peak pressures under the foot were greater in the group who had ulcerations.360 A number of studies have examined the effect of peripheral neuropathy on postural stability and gait. Studies examined both a subjective history of falling as well as biomechanical function in standing and in walking.89 Peripheral neuropathy, regardless of the cause, was demonstrated to be associated with an increased risk of falling.323 The abnormalities appear greater in standing and balance than in gait.91,351 Clinically, it is important to keep in mind that balance is a function of three factors: proprioception, which is affected by peripheral neuropathy in the lower extremities; vision; and the vestibular system. The first two factors can be damaged by complications of diabetes, namely, peripheral neuropathy and retinopathy. Therefore the physician should be aware of the risk of balance issues when both of these complications are present. Autonomic neuropathy produces a loss of normal sweating in the skin of the foot. This leads to stiff, dry, scaly skin that cracks easily because of its inflexible quality. Cracks in thickened skin, and especially in the stiff calluses on the sole, propagate into and through the dermis to open pathways for infection (Fig. 27-2). Loss of normal skin temperature regulation is also a result of autonomic neuropathy. A blunted response in diabetic extremities to increased temperature demonstrates this autosympathectomy effect and is thought to contribute to infection and injury.158 Autonomic neuropathy, as measured by blood flow variability and vasoconstrictor response, was demonstrated to be associated with foot ulceration in diabetic patients with neuropathy.162 Motor neuropathy is expressed as a loss of function and contracture of the intrinsic muscles of the foot that leads to the classic claw toe deformities (Fig. 27-3). These changes predispose to ulcerations on the dorsum of the digits. The claw toe deformity contributes to a second area of ulceration as well because it increases pressure beneath the metatarsal head as the pressure of the base of the hyperextended proximal phalanx pushes down on the metatarsal head through its intact but contracted tendons.150 Magnetic resonance imaging (MRI) in neuropathic patients has demonstrated extensive atrophy of the intrinsic foot muscles, which precedes clinical hammer toe deformity.80 Mononeuropathy, most often affecting the peroneal nerve, can produce unilateral or bilateral footdrop, which is often unrecognized until the patient has developed a fixed equinus deformity. The equinus position increases pressure on the forefoot and thus contributes to ulceration of the forefoot or the distal end of a partial foot amputation. Arteriosclerotic disease is more common and more severe in diabetic than in nondiabetic patients.275 The calcification of atherosclerotic plaques in nondiabetic patients is patchy and occurs within the intimal layer of the vessel. In contrast, the characteristic calcification in diabetic arteries is circumferential and diffusely distributed along the vessel length and occurs within the tunica media of the arteries. This produces the typical lead pipe appearance of calcified arteries on radiographs and can be seen in large and small vessels alike. It is often visible out to the vessels of the midfoot or even the toes. These calcified vessels are noncompliant and brittle. They produce artifactual elevation of Doppler pressure measurements in the lower extremities and make vascular reconstruction problematic at the time of anastomosis of a saphenous vein bypass graft into this stiff tissue. No evidence, however, indicates that calcification per se, rather than occlusion, is directly responsible for decreased perfusion in the foot.101 Much has been made of so-called small-vessel disease or microvascular changes of the diabetic foot. However, recent studies question the reality of small-vessel disease at the subarteriolar level. The occlusive nature of this supposed entity in the absence of large-vessel occlusion remains undocumented and unproved. In addition, adequate correlation has not been proved between the known changes in capillary permeability and basement membrane thickening in the diabetic foot and the physiologic events of gangrene and infection.262 Actual measurement of blood flow in diabetic patients with supposed small-vessel disease failed to show a difference when compared with control subjects with and without peripheral neuropathy from other causes; this led the investigators to conclude that toe lesions were caused by changes of neuropathy and that the lesion of “microvascular disease” remains unproved.196 Stiffness and callous formation within the skin have also been found to be factors associated with ulceration. Whether the callous formation represents a true independent variable or is another expression of pressure is not proved. Measures of plantar tissue stiffness or plantar skin hardness remain to be defined with regard to their clinical relevance.121,229,286 The thickness of plantar tissue has been suggested to be related to the magnitude of peak plantar pressures in diabetic patients,4,166 although in one study, the soft tissue thickness was not greater in control subjects than in diabetic subjects.324 Recent studies by Abboud et al3 on muscle dysfunction provide fascinating new information with regard to a dynamic component of increased foot pressure leading to ulceration. Diabetic patients were shown to have a greater period of contact pressure and a measurable delay in initiation of contraction of the tibialis anterior. The authors postulated that late firing of the ankle dorsiflexors resulted in reduced control of foot contact after heel strike.3 New study methodology continues to be consistent with the basic clinical principle that ulceration requires both pressure and neuropathy. Foot pressures alone do not predict foot ulcerations.119,243 Another mechanical factor that has been investigated concerns structural changes within the foot itself. Authors have shown a decreased plantar muscle thickness consistent with motor neuropathy, as well as changes in metatarsophalangeal (MTP) joint extension and increased MTP joint arthropathy that may be contributing to the development of ulcerations. These measurements appear to represent a structural measurement for the effects of autonomic and motor neuropathy described earlier.324 However, the relationship of static foot structure to plantar pressure and ulceration formation is yet to be fully defined.90 Biochemical changes in the periarticular tissues, especially of the small joints (e.g., in the hand and foot), have been noted to produce thickened skin, diminished range of motion, and joint contractures in diabetic patients (Fig 27-4).231 Recent studies have demonstrated similar changes producing abnormal joint mechanics in the feet and ankle.136,319 Subtalar motion is decreased in diabetic patients and, to a greater degree, in those with a previous history of ulceration than in those who have not had foot ulcers.126 It is not possible to state that this represents a cause-and-effect relationship between joint stiffness and the ulcerations. Some suggest that the limited joint mobility syndrome itself leads to increased plantar pressures.150,282,326 One study found an association with Charcot arthropathy, although it was unclear whether the limited joint mobility syndrome was an effect rather than a cause.104 Ulcerations of the diabetic foot are the single most common problem for which medical assistance is sought. The economic burden of diabetic ulcers is significant even in a multidisciplinary clinic specifically designed for efficient care of diabetic patients.15,328 The lifetime risk of developing a diabetic foot ulcer has been reported at 15%, with an annual incidence of 2%.59,355 A number of epidemiologic studies have examined risk factors for the development of diabetic ulceration. Abbott et al1 showed that a number of factors were independently related to the risk of developing a new foot ulcer: prior ulceration of the foot, abnormal neuropathy disability score, previous foot treatment, inability to feel the 10-g monofilament, reduced pulses, foot deformities, abnormal reflexes, and age. These authors concluded that the neuropathy disability score, pulse palpation, and monofilament testing were the three most important tests in routine screening, although an accurate history of prior ulceration was even more highly associated.1,2 More recent investigation by Lavery et al245 identified seven common pathways to ulceration. Factors most significantly associated with ulceration included neuropathy, deformity, callus, elevated peak pressure, peripheral vascular disease, penetrating trauma, and footwear-related issues. Several authors have shown the importance of psychosocial problems, such as alcohol addiction and divorce, as well as the duration of diabetes and poor metabolic control.65 Boyko et al60 showed that foot deformities, reduced foot perfusion, poor vision, and sensory and autonomic neuropathy were independently related to the development of foot ulcers. A second study by Reiber et al60 showed that trauma, neuropathy, and deformity were important causes of ulceration.321 These authors also found increased risk with greater body mass. However, other authors found that body mass was unrelated or a poor predictor of risk.65,92 Apelqvist et al11,14 showed that peripheral vascular disease is a high-risk factor for amputation. This is compounded by other medical problems such as nephropathy, which was measured in terms of proteinuria. The development of ulcerations was associated in these studies with age and other diabetic complications, including retinopathy. The International Working Group on the Diabetic Foot developed a system of risk classification that predicts clinical outcomes of foot lesions in diabetic patients.301 In this classification, group 0 were patients without neuropathy, group 1 were patients with neuropathy but without foot deformity or peripheral vascular disease, group 2 were patients with neuropathy and either deformity or peripheral vascular disease, and group 3 were patients with a history of foot ulceration or lower extremity amputation. A prospective case-control study showed that the classification was associated with ulceration and amputation rates.301 A principle to remember is that once an ulcer occurs in the diabetic patient, it signifies that the patient has now reached a level of peripheral neuropathy at which the feet will always be at equal or greater risk in the future. Several epidemiologic studies have documented that the presence or prior occurrence of an ulcer is one of the major risk factors for new ulceration and for amputation in the diabetic patient.1,11,14,60 Sensory neuropathy alone does not cause breakdown but acts in combination with pressure. All neurotrophic ulceration is caused by these two factors combined. Brand62 elegantly demonstrated with animal models that repetitive trauma produces tissue inflammation that then progresses to tissue necrosis, even in the absence of ischemia. Both the inflammation and necrosis was confirmed histologically. This is mechanical breakdown of the tissue. Repetitive low-level trauma, which is not excessive for a given repetition but is abnormal in the number of repetitions permitted (as a result of the lack of sensation) causes tissue breakdown by eventually exceeding the threshold of soft tissue tolerance. Brand summarized the concepts in a review that emphasized the importance of patient awareness and understanding of the “tenderized foot.” His three principles were the severity and localization of sensory loss on the foot, the magnitude of forces on the foot, and the quantity of repetition (walking distance) of the forces necessary to lead to tissue inflammation and breakdown.63 Objective scientific methods have now corroborated the assertions that ulcerations are the result of a combination of pressure and neuropathy, such as the study comparing plantar pressures in a group of diabetic patients and a group of patients with rheumatoid arthritis. Each had abnormal pressures under the foot in about the same degree and incidence, but only the diabetic patients had plantar ulceration corresponding to the presence of neuropathy, and ulceration did not occur in the patients with arthritis.268 Plantar ulcerations almost always correspond to areas of underlying bony prominence in the foot, which explains why most ulcers occur under the metatarsal heads, the medial sesamoid, and the base of the fifth metatarsal; over the proximal interphalangeal joint of claw toes; and so on (Fig. 27-5A-D). Although ulcerations can occur under calluses, which are themselves the result of pressure, the underlying pressure rather than the calluses themselves is the risk factor.110 In contrast to plantar ulcerations, which are caused by intermittent pressure, the ulcerations on the dorsum and medial and lateral sides of the foot are usually caused by constant pressure exerted by a shoe. This can produce an ulcer even more quickly, even within 1 hour of wearing a poorly fitting shoe. The surgeon must correctly recognize this as the cause of ulcers to address the issue of footwear (Fig. 27-6). Several wound classifications systems have been proposed for the diabetic foot.20,212,241,361 The classification system developed in the 1970s at Rancho Los Amigos Hospital in California by Wagner and Meggitt, otherwise known as the Wagner classification, has been the most widely used and accepted grading system for lesions of the diabetic foot (Table 27-1).82,384 Numerous authors have since worked to improve the understanding of wounds, particularly diabetic foot wounds, and much progress has been made. The Wagner classification, however, through its history and its simplicity, has provided a basis for communication and comparison among physicians and investigators in this area. The original Rancho Los Amigos classification presented the first widely referenced classification of diabetic foot lesions. However, limitations of this system exist in light of further experience. Table 27-1 Wagner Classification for Diabetic Foot Ulcerations Figure 27-7 Grade 1 ulcer under midfoot Charcot prominence. The ulcer is superficial and filled with granulation tissue. The depth–ischemia classification68 is a modification of the Wagner-Meggitt classification (Fig. 27-9). The purpose of this modified classification is to make the classification easier to use by separating the evaluation of the wound from the evaluation of the perfusion of the foot; to clarify the distinctions among the grades, especially between grades 2 and 3; and to simplify the task of correlating appropriate treatments with the proper grade of lesion. This revision of the classification of the lesions of the diabetic foot, combined with the treatment flowchart (Fig. 27-10), makes the decision-making path clearer and this information more accessible than with the Wagner algorithms.382 With the depth-ischemia classification, the soft tissue first is inspected and graded with a number grade, and then the limb and foot are evaluated for perfusion. The first step is based primarily on inspection of the foot and its wound or ulcer. Grade 1 lesions are superficial without exposure to sight or to the air of any deep structure. Grade 2 lesions are deeper by virtue of exposure of tendon or joint capsule, with or without infection. Grade 3 lesions are the deepest, composed of exposed bone, deep infection, abscess, or osteomyelitis (Fig. 27-11A and B). Determination of these three basic wound grades is based on the simple physical examination consisting of visualization and gentle probing of the wound with a sterile blunt instrument. Once the examiner has assigned a numeric grade to the foot, the second step is to assign the letter grade by evaluating the limb perfusion (see Fig. 27-9). Grade A identifies the foot without clinically significant ischemia and does not require vascular evaluation or intervention. Grade B is the widest category in the ischemia portion of the classification. This foot has diminished perfusion but is not gangrenous. Grade B feet require a screening test, such as arterial Doppler studies or transcutaneous oxygen measurements. Depending on the outcome of these screening tests, vascular reconstruction might be indicated. Grade C feet have partial gangrene, and grade D feet have complete gangrene. Grades C and D require vascular evaluation to determine the appropriate level of healing and the possible need for vascular bypass to achieve healing of that amputation. A great advance has been the University of Texas classification.241 It is the same as the depth–ischemia classification in its definitions of wound depth and separation of that from evaluation of ischemia (Table 27-2). The advantage of this classification is that it includes consideration of infection. The authors have called the presence of infection or ischemia “stages” of each grade (or depth). The stage A is neither infected nor ischemic. Stage B is infected but not ischemic. Stage C is ischemic but not infected. Stage D is both ischemic and infected. Table 27-2 University of Texas Ulcer Classification
Diabetes
Background and History
Pathophysiology
Metabolic Control
Neuropathy
Angiopathy
Structural Changes
Common Clinical Problems Associated with Diabetes
Ulceration
Classification of Diabetic Foot Ulcerations
Wagner Grade
Clinical Characteristics
0
Preulcerative or postulcerative lesion
1
Partial or full-thickness superficial ulceration (Fig. 27-7)
2
Ulceration that probes to tendon or capsule (Fig. 27-8)
3
Deep ulceration to bone
4
Partial foot gangrene
5
Whole foot gangrene
Grade
Clinical Characteristics
0
Preulcerative or postulcerative lesion
1
Partial or full-thickness superficial ulceration
2
Deep wound that involves tendon or capsule
3
Wound penetrating bone or joint
Stage
A
Clean wound
B
Nonischemic infected wound
C
Ischemic noninfected wound
D
Ischemic infected wound ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diabetes