6 Deep dry needling of the head and neck muscles
Introduction
Neck, head, and orofacial pain syndromes are among the most common problems seen in daily clinical practice. Headache is the most prevalent neurological pain disorder seen by medical doctors and experienced by almost everyone (Bendtsen & Jensen 2010). Orofacial pain from muscular origin is as prevalent as headaches (Svensson 2007).
The lifetime and point prevalence of neck pain are almost as high as low back pain. Neck pain affects 45–54% of the general population at some time during their lives (Côte et al. 1998) and can result in severe disability (Côte et al. 2000). The lifetime prevalence of idiopathic neck pain has been estimated to be between 67–71% indicating that approximately two-thirds of the general population will experience an episode of neck pain at some time during their life (Picavet et al. 2000). In a systematic review, Fejer et al. (2006) found a 1-year prevalence for neck pain ranging from 16.7% to 75.1%. Additionally, the economic burden of neck pain involves high annual compensation costs (Manchikanti et al. 2009).
Among the different primary headaches, migraine and frequent tension-type headache represent the most common forms (Bendtsen & Jensen 2006). Globally, the percentage of adults with headache is 10% for migraine, 38% for tension-type headache, and 3% for chronic daily headache (Jensen & Stovner, 2008). In the general population, the prevalence rate of cervicogenic headache is reported as 4.1% (Sjaastad & Bakketeig, 2008). The prevalence of cervicogenic headache is, however, difficult to determine, because epidemiological studies used different criteria for the diagnosis (Haldeman & Dagenais 2001). Headaches cause substantial disability for patients and their families as well as to the global society (Stovner et al. 2007). In the US, the estimated total cost in 1998 was $14.4 billion for 22 million migraine sufferers (Hu et al. 1999), whereas in Europe the estimated cost in 2004 was €27 billion for 41 million patients (Andlin-Sobocki et al. 2005).
Orofacial pain is usually and clinically associated with headaches. Its prevalence is, however, under debate, with studies showing prevalence rates between 3% and 15% in the Western population (LeResche 1997). Isong et al. (2008) determined that the prevalence of orofacial pain was 4.6% (6.3% for women, 2.8% for men).
Neck, head, and facial pain are also common clinical manifestations of subjects suffering from whiplash associated disorders (Drottning et al. 2002). Neck injuries following motor vehicle accidents comprised 28% of all injuries seen in emergency room departments in 2000 (Quinlan et al. 2004). In the USA, the incidence rate was 4.2 per 1000 inhabitants (Sterner et al. 2003), whereas the prevalence rate was 1% (Richter et al. 2000). In addition the annual costs of motor vehicle crashes during 1999–2001 were estimated in the USA at $346 billion, with $43 billion attributed to whiplash (Zaloshnja et al. 2006).
These pain syndromes have common clinical features and are usually co-morbid entities suggesting a common nociceptive pathway with sensitization mechanisms mediated through the trigeminal nucleus caudalis. The exact pathogenesis of the pain is not completely understood. Simons et al. (1999) described the referred pain elicited from trigger points (TrPs) in several muscles that can play a relevant role in the genesis of these syndromes. In this chapter we cover dry needling of TrPs in the head and neck musculature based on clinical and scientific reasoning.
Clinical presentation of TrPs in head and neck pain syndromes
Trigger points (TrPs) in headache and orofacial pain populations
In the past few years, there have been an increasing number of studies confirming the relevance of TrPs in head, neck, and face pain syndromes (Fernández-de-las-Peñas et al. 2007a). Clinicians should consider that differences in the pain characteristics of tension-type, cervicogenic, and migraine headaches implicate different structures, which may be contributing to nociceptive irritation of the trigeminal nucleus caudalis, and feature a different involvement of TrPs. For instance, tension-type headache is characterized by pressing or tightening pain, pressure or band-like tightness, and increased tenderness on palpation of the neck and shoulder muscles (ICHD-II 2004), which resemble clinical descriptions of pain from TrPs (Simons et al. 1999, Gerwin 2005). We summarize pertinent clinical and scientific evidence related to TrPs in head, neck, and face pain syndromes.
Myofascial TrPs in temporomandibular pain
There is scientific data that referred pain from masticatory muscles can be involved in orofacial pain syndromes (Svensson & Graven-Nielsen 2001). Experimental studies reproduced motor and sensory disturbances, including hyperalgesia, local, and referred pain, similar to those reported for temporomandibular pain patients after injecting irritating substances into the masseter muscle (Svensson et al. 2003a, 2003b, 2008). Svensson (2007) suggested that different muscles are involved in the pathophysiology of temporomandibular pain, such as the masseter, where for example the upper trapezius and suboccipital muscles may be more common in tension-type headaches. Nevertheless, few studies investigating the presence of TrPs in temporomandibular pain have been conducted. Wright (2000) found in a sample of 190 patients with temporomandibular pain, that the upper trapezius (60%), lateral pterygoid (50%), and masseter (47%) muscles were the most common sources of referred pain into the craniofacial region. The cheek area, ear, and forehead were the most frequently reported sites of referred pain generation. Nevertheless, this study did not include a control group and patients were not examined in a blinded fashion.
Fernández-de-las-Peñas et al. (2010) conducted a blind-controlled study where patients with myofascial temporomandibular pain and healthy controls were examined for TrPs in the neck and head muscles. They found that active TrPs in the masticatory muscles, i.e., the superficial masseter (78%), temporalis (73%), and deep masseter (72%) were more prevalent than TrPs within the neck and shoulder muscles, i.e., upper trapezius (64%), suboccipital (60%), and sternocleidomastoid (48%) muscles (Fernández-de-las-Peñas et al. 2010). This would be expected since masticatory muscle TrPs are more likely to play a role in temporomandibular pain, whereas neck and shoulder TrPs would play a greater role in headaches. In addition, TrPs in the neck and shoulder muscles may be implicated in symptoms of the neck, which are commonly seen with patients suffering from temporomandibular pain (De Wijer et al. 1999). In fact, preliminary evidence suggests that application of treatment targeted to the cervical spine was beneficial in decreasing pain intensity and pressure pain sensitivity over the masticatory muscles and in increasing pain-free mouth opening in patients with myofascial TMD (La-Touche et al. 2009).
Myofascial TrPs in tension-type headache
Tension-type headache (TTH) is a type of headache for which there is clear scientific evidence of an etiologic role for TrPs (Fernández-de-las-Peñas & Schoenen 2009). Marcus et al. (1999) reported that patients with TTH had a greater number of either active or latent TrPs than healthy controls; however, this study did not specify in which muscles TrPs were most frequently found.
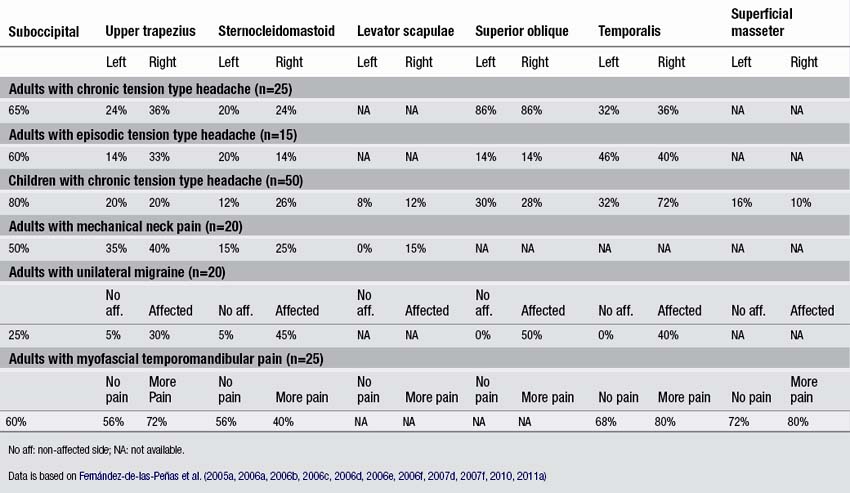
In a series of blinded-controlled studies, Fernández-de-las-Peñas et al. found that active TrPs were extremely prevalent in individuals with chronic and episodic TTH. Patients with chronic TTH have active TrPs in the extra-ocular superior oblique muscles (86%, Fernández-de-las-Peñas et al. 2005), the suboccipital muscles (65%, Fernández-de-las-Peñas et al. 2006a), the upper trapezius (50–70% Fernández-de-las-Peñas et al. 2006b, 2007b), temporalis (60–70%, Fernández-de-las-Peñas et al. 2006b, 2007c), sternocleidomastoid (50–60%, Fernández-de-las-Peñas et al. 2006b), and extra-ocular rectus lateralis muscles (60%, Fernández-de-las-Peñas et al. 2009). Additionally, patients with chronic TTH and active TrPs in these muscles exhibited more severe headaches with greater intensity, frequency, and duration than patients with chronic TTH and latent TrPs in the same muscles (Fernández-de-las-Peñas et al. 2007e). Given that temporal summation of pain is centrally mediated (Vierck et al. 1997), a temporal integration of nociceptive signals from muscle TrPs by central nociceptive neurons is probable, leading to sensitization of central pathways in chronic TTH (Bendtsen & Schoenen 2006). Couppe et al. (2007) also found a higher prevalence of TrPs in the upper trapezius muscle (85%) in patients with chronic TTH. In addition, TrPs were found in children with chronic TTH. A case series of nine 13-year old girls with TTH suggested that TrPs do play an important role in at least a subgroup of children with TTH (Von Stülpnagel et al. 2009). These girls received TrP treatments twice a week. After 6.5 sessions, the headache frequency was reduced by 67.7%, the intensity by 74.3%, and the mean duration by 77.3% (Von Stülpnagel et al. 2009). In a blinded-controlled study, Fernández-de-las-Peñas et al. (2011a) reported that in children with a mean age of 8 with chronic TTH, the suboccipital (80%), the temporalis (54%), the ocular superior oblique (28–30%), the upper trapezius (20%), and the sternocleidomastoid (12–26%) muscles harbored most TrPs. Active TrPs have been reported in episodic TTH as well, but less frequently. The most common muscles with active TrPs included the superior oblique muscle (15%, Fernández-de-las-Peñas et al. 2005), the suboccipital muscles (60%, Fernández-de-las-Peñas et al. 2006c), the sternocleidomastoid (20%), the temporalis (45%), and the upper trapezius muscle (35%, Fernández-de-las-Peñas et al. 2007d). A recent study confirmed that active TrPs are more prevalent in chronic TTH than in episodic TTH (Sohn et al. 2010). The association of active TrPs with episodic TTH does not support the hypothesis that active TrPs are always a consequence of central sensitization, since central sensitization is not as common in episodic TTH as in chronic TTH (Fernández-de-las-Peñas et al. 2006d). TrPs located in other muscles not included in these studies, such as the masseter, splenius capitis, scalene, levator scapulae muscles, may also contribute to the pain symptoms in individuals with TTH. Table 6.1 details the percentage of individuals presenting active TrPs in patients with head and neck pain syndromes discussed in the chapter.
Finally, a few studies explored the effects of the treatment of TrP in patients with chronic TTH. Moraska & Chandler (2008), in a pilot study, demonstrated that a structured massage program targeted at inactivating TrPs was effective for reducing headache pain and disability in individuals with TTH; however, this study did not include a control group. Similarly, these authors reported that the same TrP massage program improved psychological measures, particularly depression and the number of events deemed as stressful (Moraska & Chandler, 2009). Fernández-de-las-Peñas et al. (2008) developed a preliminary clinical prediction rule to identify women with chronic TTH who most likely would experience short-term favorable outcomes after TrP manual therapy. Four variables were identified for immediate success and 2 for 1-month success (Table 6.2). If all variables (4 + LR: 5.9) were present, the chance of experiencing immediate benefit from TrP treatment improved from 54% to 87.4% (Fernández-de-las-Peñas et al. 2008). However, a limitation of this study was its relatively small sample size (n=35). A second clinical prediction rule where women with chronic TTH received a multimodal therapy session identified eight variables for short-term success (Fernández-de-las-Peñas et al. 2011b). The variables are listed in Table 6.3. If five of the eight variables (5 + LR: 7.1) were present, the chance of experiencing successful treatment improved from 47% to 86.3% (Fernández-de-las-Peñas et al. 2011b). Therapeutic procedures included both joint mobilizations to the cervical and thoracic spine and soft tissue TrP therapies such as soft tissue stroking, pressure release, and muscle energy techniques applied to the neck, head and shoulder musculature: temporalis, suboccipital, upper trapezius, sternocleidomastoid, and splenius capitis muscles (Fernández-de-las-Peñas et al. 2011b). These clinical rules support the role of TrPs in the management of TTH; however, further studies validating current data are now needed.
Table 6.2 Variables identified for immediate success (top) and for 1-month success (bottom) including accuracy statistics with 95% confidence intervals for each variable (from Fernández-de-las-Peñas et al. 2008)
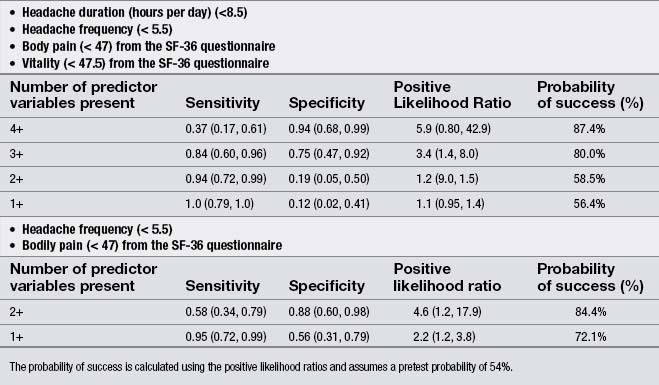
Table 6.3 Variables identified for immediate success including accuracy statistics with 95% confidence intervals for each variable success (from Fernández-de-las-Peñas et al. 2011b)
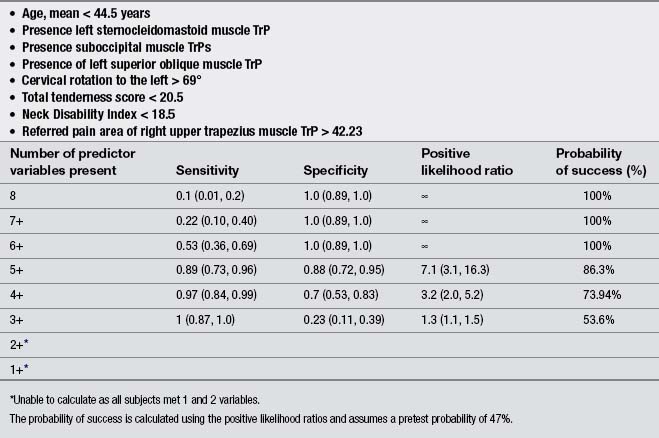
There are a small number of studies investigating the effects of dry needling and TTH. De Abreu Venâncio et al. (2008) compared the effects of TrP injections using lidocaine to TrP dry needling in the management of headaches of myofascial origin. They found that TrP dry needling was equally effective for decreasing the intensity, the frequency and the duration of the headache, and for the use of rescue medication than injections using lidocaine alone or combined with corticoids. The same authors also reported that TrP dry needling was equally effective as botulinum toxin A for decreasing the intensity, the frequency and duration of the pain, but less effective for the use of rescue medication (De Abreu Venâncio et al. et al. 2009). These results are similar to those by Harden et al. (2009) who reported that patients who received botulinum toxin A injections over active TrPs experienced reductions in headache frequency at short term, but the effects dissipated by week 12. Headache intensity also revealed a decrease in the botulinum toxin A group, but not in the control group (Harden et al. 2009).
Myofascial TrPs in migraine
TrPs have been also found in patients with migraine. In unilateral migraine, active TrPs in the upper trapezius (30%), sternocleidomastoid (45%) and temporalis (40%) muscles were located only ipsilateral to migraine attacks as compared to the non-symptomatic side (Fernández-de-las-Peñas et al. 2006e). TrPs in the extra-ocular superior oblique muscle (50%) were present in the symptomatic, but not in the non-symptomatic side (Fernández-de-las-Peñas et al. 2006f). A study of 92 patients with bilateral migraine showed that 94% exhibited TrPs in the temporalis and suboccipital muscles compared with 29% of controls (Calandre et al. 2006). The number of TrPs was related to the frequency of migraine headaches and the duration of the disease (Calandre et al. 2006).
Referred pain from active TrPs reproduced the pain features of migraine headache, although patients were examined when they did not have a headache (Fernández-de-las-Peñas et al. 2006e). Nevertheless, an association of TrPs with migraine does not constitute a causal relationship. The presence of TrPs indicates that peripheral nociceptive input from TrPs into the trigeminal nucleus may act as a migraine trigger. A link between pain generators of the neck, head and shoulder muscles and migraine attacks may be the activation of the trigeminal nerve nucleus caudalis, and hence the activation of the trigeminovascular system. In such instance, TrPs located in any muscle innervated by the trigeminal nerve or the upper cervical nerves may be considered as ‘irritative thorns’ that can precipitate, perpetuate or aggravate migraine. Obviously, other triggers also exist for migraine.
Evidence supporting a triggering role of TrPs in migraine comes from the resolution of migraine headache by treating TrPs in neck and shoulder muscles with lidocaine or saline injections (Tfelt-Hansen et al. 1981; Calandre et al. 2003). In addition, inactivation of active TrPs in migraine patients not only reduced the electrical pain threshold in the headache area of pain referral, but also reduced the number of headache attacks over the 60 days of the treatment period (Giamberardino et al. 2007). García-Leiva et al. (2007) reported that TrP injection with ropivacaine (10mg) was effective for reducing frequency and intensity of migraine attacks.
Myofascial TrPs in other headaches
TrPs have been also investigated in other headaches, such as cervicogenic and cluster headache. Jaeger (1989) found in a cohort of eleven individuals with cervicogenic headache that all patients showed at least three TrPs on the symptomatic side, especially in the sternocleidomastoid and temporalis muscles. Further, those patients who were treated reported a significant decrease in their headache frequency and intensity, which supports the role of TrPs in headache pain perception in this headache disorder (Jaeger 1989). Roth et al. (2007) described a case report where pain from TrPs in the sternocleidomastoid muscle mimicked the symptoms of cervicogenic headache. Although TrPs can obviously contribute to cervicogenic headache pain, it seems that this headache is mainly provoked by referred pain from the upper cervical joints (Aprill et al. 2002), rather than referred pain elicited by muscle TrPs. Nevertheless, this conclusion may be related to the fact that TrPs have not been properly studied in cervicogenic headache pain. Therefore, further studies are required to elucidate the role of TrPs in this headache disorder.
Calandre et al. (2008) studied the presence of TrPs in 12 patients with cluster headache. All patients showed active TrPs reproducing their headache. In this case series, TrP injection were successful in about 80% of the patients. The authors suggested that in some patients TrPs may trigger cluster headaches (Calandre et al. 2008). Ashkenazi et al. (2010) analyzed, in a systematic review, studies on peripheral nerve blocks and TrP injections for headache disorders and reported few controlled studies on the efficacy of peripheral nerve blocks and almost none on the use of TrP injections. These authors concluded that the technique, the type and the doses of the anesthetics used for nerve blockade varied greatly among studies, but in general, the results were positive. Nevertheless, this finding should be considered with caution due to the limitations of the included studies (Ashkenazi et al. 2010).
Trigger points (TrPs) in neck pain populations
Neck pain can have a traumatic or an insidious onset. A traumatic onset is seen for example following a whiplash injury. An example of an insidious cause is mechanical neck pain, which is defined as generalized neck or shoulder pain with symptoms provoked by neck postures, by movement, or palpation of the cervical muscles. Fernández-de-las-Peñas et al. (2007f) found that patients with mechanical insidious neck pain exhibited active TrPs in the upper trapezius (20%), the sternocleidomastoid (14%), suboccipital (50%) and levator scapulae (15%) muscles (Table 6.1). The presence of TrPs in the upper trapezius muscle was associated with the presence of cervical joint dysfunction at the levels of the C3 and C4 vertebrae in individuals suffering from neck pain (Fernández-de-las-Peñas et al. 2005b). Therefore, clinicians should include the assessment and treatment of joint hypomobility in the management of TrPs in individuals with mechanical neck pain (Fernández-de-las-Peñas 2009).
There is some evidence of the effectiveness of TrPs manual techniques in the management of mechanical neck pain. For instance, Montañez-Aguilera et al. (2010) reported that an ischemic compression technique was effective in the treatment of TrPs in a patient with neck pain. Bablis et al. (2008) found that the application of Neuro Emotional Technique, which is a technique incorporating central and peripheral components to alleviate the effects of distressing stimuli, may be effective for reducing pain and mechanical sensitivity over TrPs in patients with chronic neck pain. Ma et al. (2010) demonstrated that the effectiveness of a miniscalpel-needle release was superior for reducing pain in patients with TrPs in the upper trapezius muscle than acupuncture needling treatment or self neck-stretching exercises alone. Further studies are needed to confirm clinical relevance of TrP treatment in the course of mechanical insidious neck pain.
TrPs have been also associated to neck pain of traumatic origin, such as whiplash-associated neck pain (Dommerholt et al. 2005, Dommerholt 2005, 2010). Schuller et al. (2000) found that 80% of 1096 individuals involved in low-velocity collisions reported muscle pain. In a review of the literature, Fernández-de-las-Peñas et al. (2003) found that the muscles most commonly affected by TrPs were the scalene muscles (Gerwin & Dommerholt 1998), the splenius capitis, upper trapezius, posterior neck, sternocleidomastoid (Baker 1986) and pectoralis minor muscles (Hong & Simons 1993). Ettlin et al. (2008) reported that semispinalis capitis muscle TrPs were more frequent in patients with whiplash-associated neck pain (85%) than in patients with non-traumatic neck pain (35%) or fibromyalgia (57%). TrPs in the upper trapezius (70–80%), levator scapulae (60–70%), sternocleidomastoid (40–50%), and masseter (20–30%) muscles were similar among these pain groups. The presence of TrPs in individuals with WAD can be related to the fact that these patients usually exhibit reduced cervical stability, muscle inhibition and hyperirritability of the cervical muscles (Headley 2005).
Finally, a few studies have demonstrated the effects of TrP inactivation in patients with whiplash-associated neck pain. Freeman et al. (2009) showed that infiltrations of 1% lidocaine into TrPs in the upper trapezius were effective in the short-term for increasing cervical range of motion and pressure pain thresholds in individuals with chronic whiplash-associated pain. Carroll et al. (2008) reported that injections of botulinum toxin type A over cervical TrPs decreased pain in patients with chronic whiplash-related neck pain. A randomized controlled clinical trial is currently being planned with the aim to demonstrate the effects of dry needling in patients with chronic whiplash-associated neck pain (Tough et al. 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree