Decision Making and Surgical Techniques for Treating Intercalary Nerve Deficits: Autograft, Allograft, and Conduits
Mark C. Shreve
Peter J. Evans
INTRODUCTION
Intercalary nerve deficits can commonly result from upper extremity trauma or resection of neuromas in continuity or tumors. If left untreated, these nerve deficits can leave a sensory or motor deficit in the patient leading to long-term disability, loss of productivity, pain, and impaired quality of life
(1,2). Mechanisms of injury vary from crushing, stretching, laceration or transection, ischemia, and/or metabolic causes. Nerve injuries continue to be a challenging and complex clinic problem, and selecting the proper treatment is difficult given the variable presentation of injury pattern and length of deficit. The literature is quite varied in regard to indications and choices for nerve repair, ranging from direct tension-free repair to autograft, allograft, or conduit repairs.
(1,2). Mechanisms of injury vary from crushing, stretching, laceration or transection, ischemia, and/or metabolic causes. Nerve injuries continue to be a challenging and complex clinic problem, and selecting the proper treatment is difficult given the variable presentation of injury pattern and length of deficit. The literature is quite varied in regard to indications and choices for nerve repair, ranging from direct tension-free repair to autograft, allograft, or conduit repairs.
In treating peripheral nerve injuries, the goal is to provide a tension-free direct repair. However, this exact clinical situation is relatively rare and also depends on whether the repair to the nerve is acute or chronic (3). Once a nerve is injured, the ends will retract to some degree and thus require a certain amount of stretch or elongation to directly repair the ends. This degree of elongation leads to a variable amount of decreased blood flow at the repaired ends, with some studies showing at least a 50% decrease in blood flow with nerve elongation over 10% and even mechanical suture pullout at more than 15% elongation (3,4). Millesi and others have shown clearly that when a direct tensionless repair is not attainable, better results can be obtained with nerve grafting under no tension (5,6,7,8).
Nerve injuries were originally classified by Seddon (9,10) into neuropraxia, axonotmesis, and neurotmesis. Sunderland (11) further modified Seddon’s classification to include five degrees of nerve injury that helped predict outcomes after nerve injury, ranging from first-degree injury with no axonal loss equivalent to neuropraxia to fifth-degree injury with axonal discontinuity and disruption of the endo-, peri- and epineurium equivalent to neurotmesis. Later et al. (12), described a sixth-degree injury, in which the peripheral nerve at any given point along the injury zone contains varying degrees of nerve injury between the separate fascicles with some demonstrating minimal second- and third-degree injuries while others demonstrate more severe fourth- and fifth-degree injuries. This leads to a varied topography of nerve injury that must be recognized and resected to obtain a healthy level of nerve fascicle available for repair. Suturing of scarred nerve ends provides limited value as scar inhibits revascularization, axonal regeneration, and Schwann cell migration. Resection of nerve ends until discrete pouting fascicular bundles become evident is critical in preparing for reconstruction (13).
When a tensionless repair is not able to be achieved, then the gold standard for segmental defects has traditionally been autologous nerve grafting (2). Autografts are low cost and have a proven track record, and there is no risk of disease transmission. However, donor site morbidity with numbness, neuroma formation, and scarring; increased operative time for harvest; as well as having a limited supply of available nerve donor tissue has prompted many to seek alternatives to autografts. Importantly, clinical outcomes of nerve grafting demonstrate an S3/M3 or better recovery in only 40% to 60% of patients, indicating that there is significant room for improvement in current techniques of neurorrhaphy and nerve grafting.
A successful series of autogenous vein nerve conduits(14) and biodegradable polyglycolic acid tubes (15) used to bridge sensory defects of less than 3 cm encouraged usage of conduits in peripheral nerve repair in the mid 1990s. Surgeons began using nerve conduits of up to 3 cm, based mainly on the work by Strauch and colleagues (16) who demonstrated in a rabbit peroneal nerve excellent growth and regeneration in autogenous vein conduits in defects less, but not greater, than 3 cm. Then, Weber et al. (17) found 0% failure in clinical gaps less than 5 mm and 34% failure in gaps greater than 5 mm, but recommended conduits for larger gaps not supported by data presented. Mackinnon and Dellon (15) found poor resolution in pain in gaps of 5 to 30 mm in length, and Wangensteen and Kalliainen (18) found 31% of their patients requiring revision in gaps 2.5 to 20 mm in length. Therefore, given these clinical results, we limit conduit repairs to gaps less than 5 mm in length.
Nerve allografts were first used in the late 1800s, but with time it was found that failures were due to immunogenicity that necessitated immunosuppression (19,20). However, more recently there is renewed interest in nerve allografts with the advent of processed nerve allografts, which create nonimmunogenic, acellular allografts, devoid of inhibitor chondroitin sulfate proteoglycans, and contain beneficial characteristics of nerve autograft (physical macrostructures, three-dimensional microstructural scaffold, and original laminin-coated endoneurial tubes) (21). Processed nerve allograft has inherent limitations as they are absent of endogenous Schwann cells and viable microvasculature and therefore available in a maximum diameter of 4 to 5 mm and 7 cm in length.
INDICATIONS/CONTRAINDICATIONS
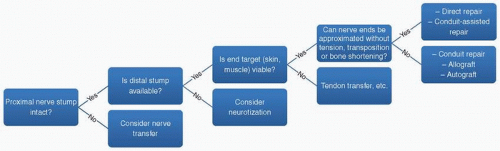
In determining whether to perform a direct repair, or utilize other techniques, we use the following algorithm (Fig. 18-1). First, if the proximal nerve stump is not available then a nerve transfer should be considered. If the proximal stump is intact, next determine if the distal nerve stump is available and if not, consider muscular neurotization. If the distal stump is available, then it should be determined if the end target (i.e., muscle or skin) is normal and healthy. If not, then a procedure such as a tendon transfer might be better utilized.
Next it should be determined if the nerve ends, after adequate stump preparation, can be approximated without undue tension, transposition, or bone shortening, under the full range of joint motion. A commonly used technique to determine if there is “too much” tension is to place a single 8-0 nylon suture in the two nerve ends, and if this is able to hold the two together, then tension is not excessive and a primary direct repair can be performed, or we prefer a conduit-assisted repair (CAR). If unable to be repaired without tension, the subsequent gap should be repaired with an autograft, allograft, or conduit, depending on the gap length.
Despite best intentions, variability exists in the injured wound environment, healing response, and surgeon microsurgical skill, and we believe that by use of a CAR, nerve repair is easier and may result in improved nerve regeneration. CARs use fewer sutures, decrease tension, avoid crumpling with misalignment of fascicles, may limit axon escape and the amount of scar invasion, and improve nerve gliding. Conduits available commercially are made of collagen, polyglycolic acid, polycaprolactone, and porcine submucosa, and have varying degrees of permeability and have varying degrees of rigidity. This rigidity prevents collapsing and kinking, which is a problem with autogenous vein conduits. However, problems have been reported with conduits made with polyglycolic acid and polycaprolactone (22). Limitations of polyglycolic acid conduits are their high rate of degradation, acidic degradation products, and low solubility. Limitations of polycaprolactone conduits include its high rigidity necessitating immersion in saline prior to use, reports of needle breakage with application necessitating larger caliber suture usage, and severe foreign body reactions and early collapse (22).
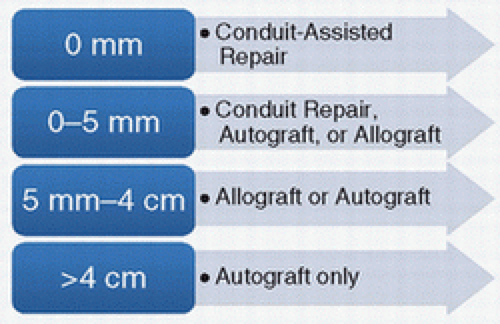
Treatment of the nerve gap can be outlined in Figure 18-2. For nerve gaps of 0 to 5 mm, we prefer to utilize a synthetic conduit or allograft for bridging the defect in digital nerves and use nerve allograft for mixed nerves, utilizing a 5- to 10-mm graft to minimize tension. For gaps from 5 mm to 4 cm, we utilize allograft, but autograft is also a standard alternative with consideration for operative time and morbidity. For defects greater than 4 cm, autograft is preferred at this time, despite the availability of allograft up to 7 cm.
PREOPERATIVE PREPARATION
The preoperative preparation will vary widely with the type of injury or indication for surgery, age and health status of the patient, size of nerve defect, and the size of nerve being repaired. Any concomitant injuries should be fully investigated and repairs planned. Any bone, tendon, or vascular injuries should be concomitantly addressed. Nerve injuries can be due to direct penetrating trauma from objects such as a knife or piece of broken glass or metal, and these injuries correlate highly with a nerve transection if a sensory or motor loss is detected. Nerve injuries can also be due to gunshot wounds or open or closed fractures, and the continuity of the nerve in question may or may not
be transected. This situation is less clear as to early or late exploration and repair. Often the zone of injury to the nerve is underappreciated and evolves over time. In the situation of early exploration of a blast injury for fixation of fractures or vascular repair, it is helpful to provisionally suture the ends of severed nerve together or at least mark the nerve ends with suture to ease later identification and reconstruction. Each injury is unique and requires a differing treatment strategy.
be transected. This situation is less clear as to early or late exploration and repair. Often the zone of injury to the nerve is underappreciated and evolves over time. In the situation of early exploration of a blast injury for fixation of fractures or vascular repair, it is helpful to provisionally suture the ends of severed nerve together or at least mark the nerve ends with suture to ease later identification and reconstruction. Each injury is unique and requires a differing treatment strategy.
Preoperative sensibility, motor function, subjective pain, dysesthesias, and sudomotor function should be evaluated and recorded. Sensibility can be adequately recorded with both static and moving two-point discrimination testing. Motor strength of involved muscle should be recorded. Depending on the acuity of the injury, electrodiagnostic testing can be helpful in recording nerve recovery and regeneration and can be helpful in distinguishing between a neuropraxic or axonotmetic and neurotmetic injuries. These studies are only helpful after at least 3 weeks to allow for wallerian degeneration to occur, in which neuropraxic injuries will show a conduction block only, unlike more severe injuries.
If considering a nerve autograft, it is important to discuss with the patients that they may have a complicating neuroma, and they will all expect a sensory loss in the dermatomal distribution supplied by the nerve being used. For elective cases, it is helpful to perform a preoperative nerve block of the donor nerve to demonstrate this expected area of anesthesia after harvest.
When performing a nerve repair or reconstruction, it is extremely important to ensure that the proper equipment is available. A pneumatic tourniquet is helpful for a bloodless field. An operating microscope has been shown to be superior to loupe magnification for microsurgical nerve repairs or reconstructions (23). A supply of 8-0, 9-0, and 10-0 monofilament sutures with a large radius of curvature is preferred for neurorrhaphy (versus vascular repair) and a taper or reverse cutting (but not spatula) point is optimal. Depending on the situation, intraoperative nerve stimulators are helpful, and ensure an adequate supply and size of nerve conduits and/or allograft nerve.
TECHNIQUE
Peripheral nerve repairs or reconstructions can be performed with the patient under general, regional, or even local anesthesia. However, if regional or local anesthesia is used, it is important that the patient be cooperative as intraoperative motion is to be avoided. Typically, general anesthesia is preferred given the prolonged operative time required for nerve reconstructions or repairs.
Conduit-Assisted Repair
An appropriately selected surgical approach is used to obtain access to the injured nerve. Prior lacerations should be incorporated in the incision if they provide adequate access to the nerve injury site. For digital nerve repair, Brunner zigzag incision is commonly used in the finger and hand. Thick skin flaps should be raised where necessary.
As with all nerve repairs, the initial injury or insult to the tissues will determine the quality and area of nerve injury. Lacerations should be explored and depending on the location any concomitant tendon or vascular injuries should be identified and addressed. These repairs are beyond the scope of this chapter. But in general any flexor or extensor tendon or digital arterial repairs should be performed prior to the repair of the nerve.
It cannot be underestimated how critical it is to perform an adequate debridement of the nerve ends in order to expose healthy, pouting axons and freely bleeding epineurial and endoneurial vessels. This should be initiated under loupe magnification and completed under the microscope. It is our contention that it is rare that even a sharp injury provides a tensionless repair, especially when explored several days post injury, and after adequate debridement a gap is always present. Neurolysis should include mobilizing the proximal and distal segments to lessen the gap distance and tension on the repair.
As with all nerve repairs, proper alignment of the fascicles is necessary. Several techniques can be used to help assure that the fascicles are best directed to the right location from proximal to distal. Direct visualization of the nerve fascicular patterns and topography can be used to properly align the nerve ends. Surface vessels can also be used on larger more proximal nerves. We have found more complex techniques such as nerve stimulation and intraoperative histologic staining, although useful and proven valuable, to be too cumbersome intraoperatively for routine nerve repairs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree