Conditions That Commonly Simulate Primary Neoplasms of Bone
Adequate consideration of all the reactive, traumatic, infectious, metabolic, congenital, and other conditions of bone that may simulate benign or malignant neoplasms is not within the scope of this book. Many of these conditions are recognized clinically and radiographically, and the surgical pathologist is not involved in making the diagnosis. The purpose in this chapter is to indicate the types of problems that are encountered and to document briefly some of those most often seen in material sent for consultation from other pathologists. Among the conditions that are not discussed are pseudotumors of bone in hemophiliac patients and hydatid disease of bone that produces a severe problem rarely seen in the United States.
METASTATIC CARCINOMAS
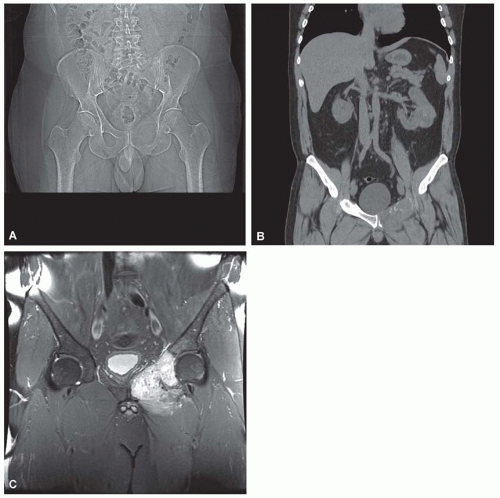
Metastatic deposits from carcinomas are by far the most common malignant tumors affecting the skeleton. Although the correct diagnosis is usually obvious when the clinical history is considered, it is often unsafe to assume that any given skeletal lesion or lesions are necessarily related to the proved carcinoma. For example, the punched-out areas of destruction characteristic of myeloma may be mistaken for areas of lytic metastatic deposits. Metastatic carcinoma is especially likely to be a diagnostic problem when only one skeletal lesion is found and no primary tumor is known. A destructive process secondary to hypernephroma is particularly likely to simulate a primary lesion of bone because this cancer tends to produce a clinically solitary metastatic lesion, the cells often show pronounced spindling, and the primary tumor is in an obscure location. Carcinomas may invade bone by direct extension.
PHYSICAL FINDINGS
The osseous lesions of metastatic carcinoma may closely simulate a primary malignant tumor. The most prominent symptoms are pain, with or without swelling, and those resulting from pressure on neighboring structures or from pathologic fracture. Systemic symptoms of a malignancy may or may not be present.
RADIOGRAPHIC FEATURES
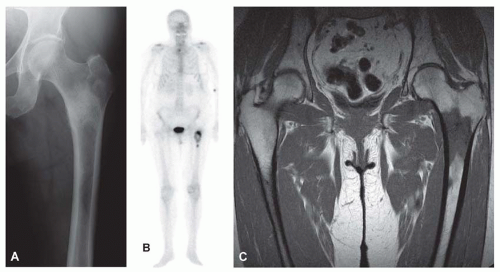
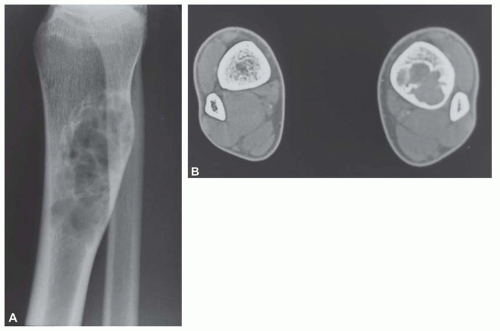
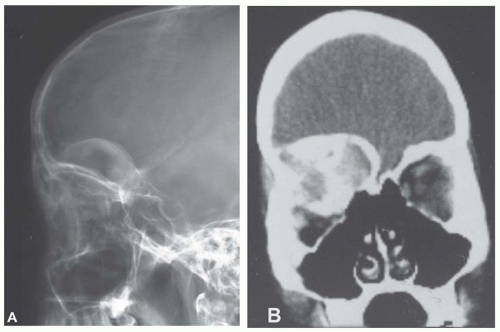
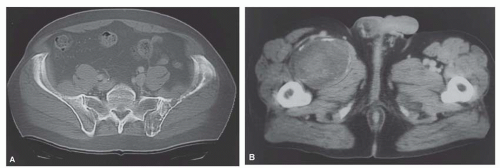
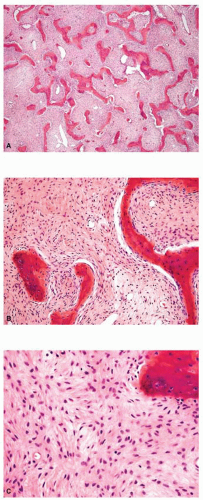
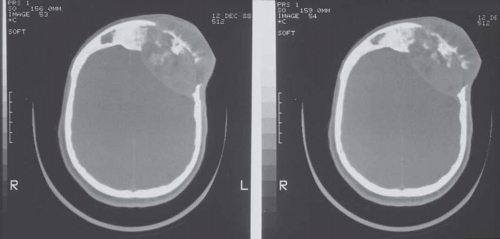
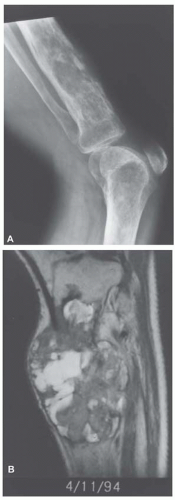
Metastatic tumors usually produce irregular destruction of bone indicative of their malignant quality. Although most such lesions are osteolytic, many metastatic deposits from carcinoma of the prostate and occasionally those from other tumors are osteoblastic. The presence of a large, purely lytic destructive lesion, which may have an aneurysmal dilatation, strongly suggests the possibility of metastatic renal cell carcinoma. Radioactive bone scans may show involvement of other skeletal sites. Magnetic resonance imaging, especially with involvement of the vertebrae, may show more extensive disease than is obvious clinically. Computed tomograms may similarly show the occult primary tumor, such as a hypernephroma (Figs. 26.1, 26.2, 26.3 and 26.4).
GROSS PATHOLOGIC FEATURES
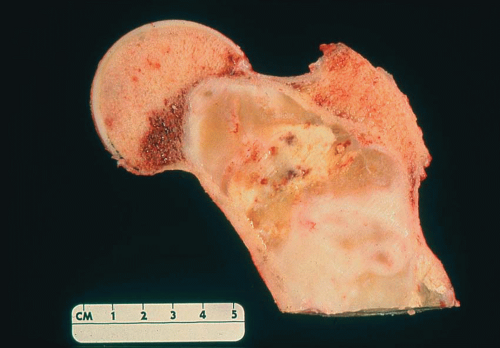
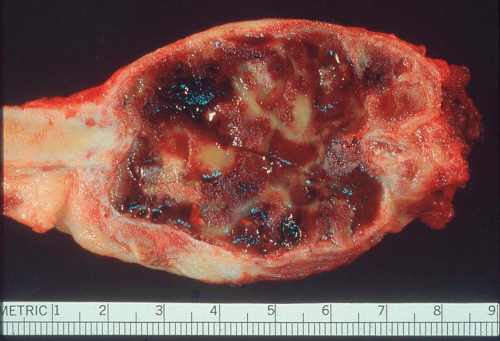
Carcinomas metastatic to bone do not have gross diagnostic characteristics. The lesions vary from those that are fibrotic because of a desmoplastic reaction produced by the tumor to those that are extremely soft and mushy. The osteoblastic metastatic lesions seen so often in prostatic carcinoma are very dense and relatively characteristic. Rarely, bone that may result
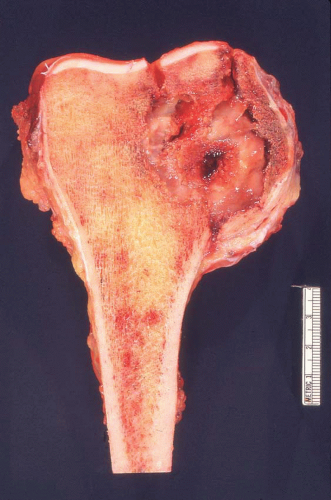
from reaction to a deposit of metastatic carcinoma is confusingly similar to that produced by osteosarcoma (Figs. 26.5, 26.6 and 26.7).
from reaction to a deposit of metastatic carcinoma is confusingly similar to that produced by osteosarcoma (Figs. 26.5, 26.6 and 26.7).
HISTOPATHOLOGIC FEATURES
Most often, the patient presenting with metastatic carcinoma to the skeleton has a known primary neoplasm. A biopsy may be performed just to confirm the presence of skeletal metastasis. Fine-needle aspiration is an excellent method for documenting metastatic disease. It is important to compare the biopsy specimen with the previous primary neoplasm if available.
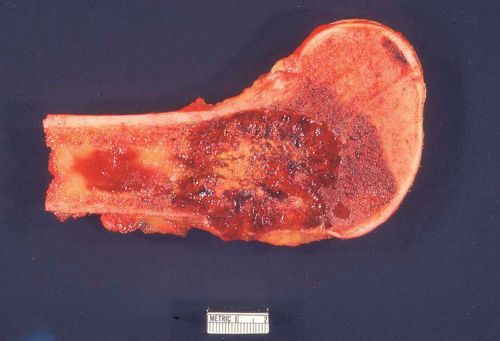
Most metastatic carcinomas are histologically obvious. Most metastatic adenocarcinomas and squamous cell carcinomas do not present diagnostic difficulties. However, when the skeletal lesion is the only sign of carcinoma, the pathologist may be in a position to guide the clinician for a search of the primary neoplasm. This exercise may be of more than academic interest. Some metastatic carcinomas, such as carcinoma of the breast, may respond to treatment, and the patient may have prolonged survival. It is obviously important to correctly identify a prostatic carcinoma so that appropriate hormonal therapy can be administered. However, a patient with a metastasis from the lung or a kidney usually has a poorer prognosis (Figs. 26.8, 26.9, 26.10, 26.11 and 26.12).
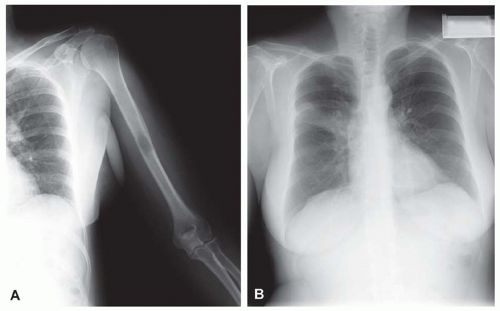
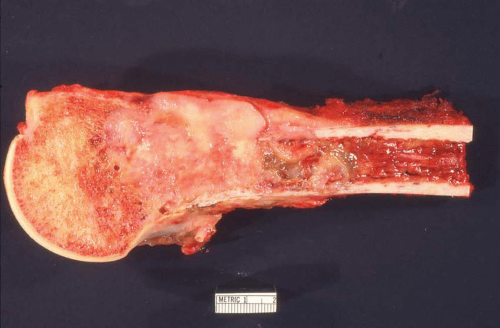 Figure 26.7. Metastatic adenocarcinoma from the lung forms a destructive mass in the proximal humerus. The mass also was involved by chronic lymphocytic leukemia/lymphoma. |
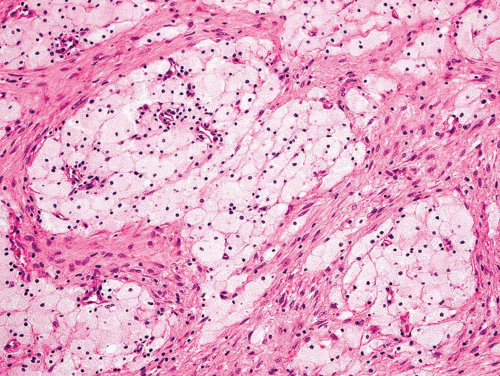
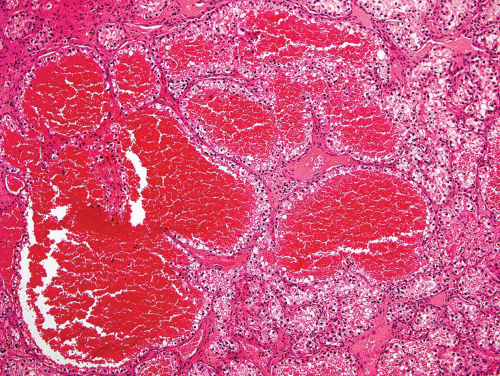 Figure 26.8. This metastatic renal cell carcinoma illustrates how these tumors are oftentimes quite vascular. |
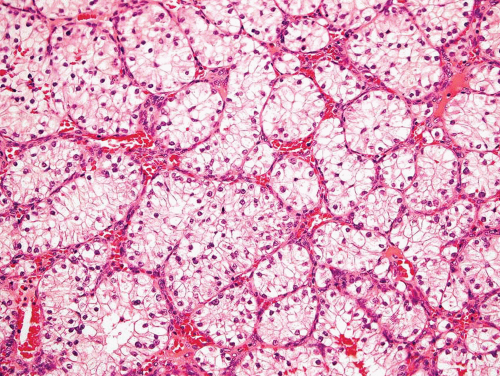 Figure 26.9. Typical appearance of metastatic renal cell carcinoma with clear cells in an organoid pattern. |
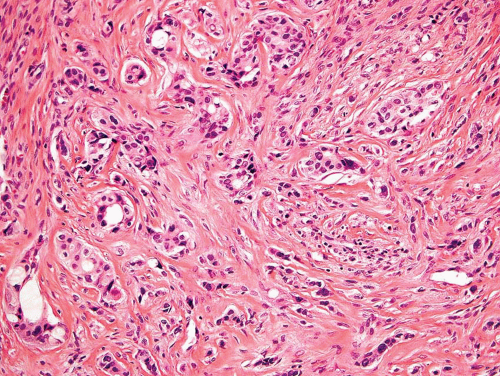 Figure 26.10. Metastatic adenocarcinoma from a breast primary tumor. The tumor is associated with dense fibrous tissue. Glandular differentiation is easily recognizable. |
With some malignancies, the pathologist can accurately pinpoint the primary site. Metastatic carcinoma from the thyroid, metastatic hepatocellular carcinoma, metastatic clear cell carcinoma, and others are so characteristic that a definite diagnosis can be made (Figs. 26.8, 26.9 and 26.10). However, with undifferentiated adenocarcinomas or squamous cell carcinomas, the pathologist can only suggest possible primary sites. Immunoperoxidase stains have become increasingly important as an adjunct in pointing toward a primary site. An example is the use of immunoperoxidase stains for prostate-specific antigen and prostatic acid phosphatase in confirming a diagnosis of prostatic carcinoma.
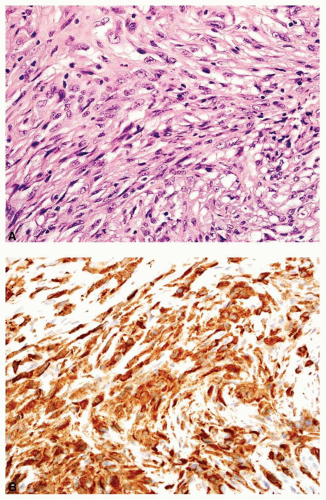
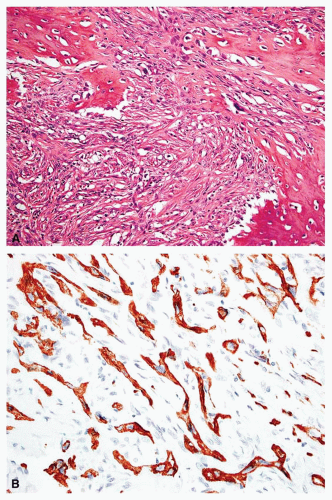
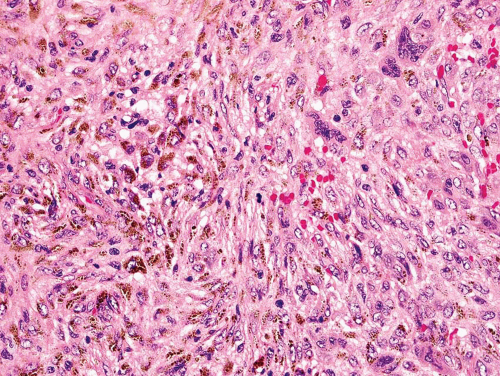
Rarely, a metastatic carcinoma is spindled and may simulate the appearance of a sarcoma. Most spindling
carcinomas have plump cells, and, in the appropriate age group, this possibility should be considered whenever a diagnosis of a primary sarcoma of bone is entertained. Sampling of the specimen may show obvious epithelial differentiation. This problem is especially common with metastatic hypernephromas that may be sarcomatoid. Immunoperoxidase stains may show the epithelial characteristics of the tumor in question (Figs. 26.11 & 26.12). However, not all sarcomatoid carcinomas show epithelial differentiation, and some sarcomas may be positive for keratin. It is important to clinically rule out the possibility of a sarcomatoid carcinoma, especially of the kidney, before definitive surgery is performed for a presumed sarcoma in an adult. In spite of extensive search, however, the kidney tumor may not become apparent.
carcinomas have plump cells, and, in the appropriate age group, this possibility should be considered whenever a diagnosis of a primary sarcoma of bone is entertained. Sampling of the specimen may show obvious epithelial differentiation. This problem is especially common with metastatic hypernephromas that may be sarcomatoid. Immunoperoxidase stains may show the epithelial characteristics of the tumor in question (Figs. 26.11 & 26.12). However, not all sarcomatoid carcinomas show epithelial differentiation, and some sarcomas may be positive for keratin. It is important to clinically rule out the possibility of a sarcomatoid carcinoma, especially of the kidney, before definitive surgery is performed for a presumed sarcoma in an adult. In spite of extensive search, however, the kidney tumor may not become apparent.
Metastatic carcinoma can also cause confusion because of other histologic features. Some metastatic carcinomas produce a large amount of reactive new bone formation. Occasionally, it may be difficult to know whether the bone is produced by the tumor or is reacting to it. As indicated previously, some osteosarcomas may appear epithelial. Hence, this problem can be very difficult. Some metastatic carcinomas produce reactive osteoclastic proliferation, and the appearance may simulate that of a giant cell tumor.
TREATMENT
The treatment of patients with skeletal metastases is becoming increasingly important. For patients with certain carcinomas, especially those from the prostate and breast, both medical and surgical hormonal therapy are beneficial. Carcinoma metastatic from the thyroid may be controlled for prolonged periods with the use of radioactive iodine. Orthopedic surgical procedures in combination with radiation or other therapy are often of much value in the management of carcinoma metastatic to the skeleton. Because patients with metastatic carcinoma are living longer, they will more likely receive aggressive treatment for incipient or actual pathologic fractures.
PROGNOSIS
As indicated above, the prognosis for metastatic carcinoma depends on the primary site. A patient with metastatic renal cell carcinoma whose primary tumor had been removed previously may live for a long time. However, patients with renal cell carcinoma presenting with metastatic skeletal disease have a poorer prognosis.
FIBROUS LESIONS
Various benign fibrous proliferations can simulate primary neoplasms of bone.
METAPHYSEAL FIBROUS DEFECT
Metaphyseal fibrous defect, fibroma, nonossifying fibroma, and fibrous cortical defect all refer to the same histopathologic process in bone. The spontaneous resolution of most metaphyseal fibrous defects and their relationship to the growing portions of bones support the concept that they represent faulty ossification rather than neoplasm. Although the term metaphyseal
fibrous defect is preferred, the term fibroma is used interchangeably. Radiographic evidence of small cortical defects may be found in approximately one-third of growing children, most commonly in the distal femur. Few of these lesions pose a significant diagnostic problem or produce enough symptoms to require surgery. A few fibroblastic masses, which histologically are indistinguishable from the innocuous ones, continue to grow and may produce pathologic fracture of even a major tubular bone. Patients may have multiple fibrous defects in one or more extremities.
fibrous defect is preferred, the term fibroma is used interchangeably. Radiographic evidence of small cortical defects may be found in approximately one-third of growing children, most commonly in the distal femur. Few of these lesions pose a significant diagnostic problem or produce enough symptoms to require surgery. A few fibroblastic masses, which histologically are indistinguishable from the innocuous ones, continue to grow and may produce pathologic fracture of even a major tubular bone. Patients may have multiple fibrous defects in one or more extremities.
When there are several metaphyseal fibrous defects, the patient may have other problems and may have Jaffe-Campanacci syndrome, as described by Mirra and colleagues.
Despite the innocuous clinical behavior of metaphyseal fibrous defect, its component of benign multinucleated cells still frequently results in its being erroneously considered a giant cell tumor of bone.
The so-called periosteal desmoid appears to be a hypocellular variant of the group of fibrous defects. The term periosteal desmoid is an unfortunate one, suggesting, as it does, an aggressive process. The lesion is situated on the posteromedial aspect of the lower end of the femur and probably results from an avulsive injury to the insertion of the aponeurotic sheath from the extensor tendon of the adductor magnus muscle; hence, the term avulsive cortical irregularity is preferred.
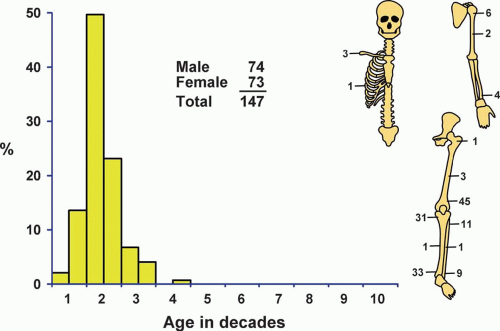 Figure 26.13. Distribution of metaphyseal fibrous defect according to age and sex of the patient and site of the lesion. |
Incidence
Although metaphyseal fibrous defects are uncommon in a surgical practice, their true incidence is much greater because most patients with these defects never undergo surgery. There were a total of 147 cases in the Mayo Clinic files (Fig. 26.13).
Sex
There was a slight male predominance.
Age
In its classic form, metaphyseal fibrous defect is almost exclusively a disease of childhood and adolescence. The oldest patient in this series was 37 years old. Sixteen patients were older than 20 years, and approximately 73% were in the second decade of life.
Localization
Every lesion in this series was in a long bone except for three involving the clavicle and one involving the rib. In the long bones, almost all lesions were in the metaphysis. Seven lesions involved the diaphysis: three in the femur, two in the humerus, and one each in the tibia and fibula. This probably is related to the growth of the bone, which pulls away from the stationary lesion.
Four patients had two lesions each. Two patients each had lesions of the tibia and fibula, and two patients had lesions of the tibia and femur. Four patients had polyostotic involvement with metaphyseal fibrous defects. One of these patients had skin pigmentation similar to that described in the Jaffe-Campanacci syndrome. Another patient had hemangiomas of soft tissue in addition to the skin pigmentation.
Symptoms
Metaphyseal fibrous defect of bone is commonly silent clinically, and it is discovered incidentally when a region is studied radiographically for unrelated reasons. Local pain, usually of short duration, is sometimes produced and may be related to small pathologic fractures. Occasionally, a patient presents with a pathologic fracture.
Physical Findings
Physical examination is of little value in diagnosing metaphyseal fibrous defect of bone. In rare instances, slight swelling may be observed if the affected bone is near the surface of the body. Occasionally, the fracture may be a compound one. As mentioned previously, two patients showed skin pigmentation.
Radiographic Features
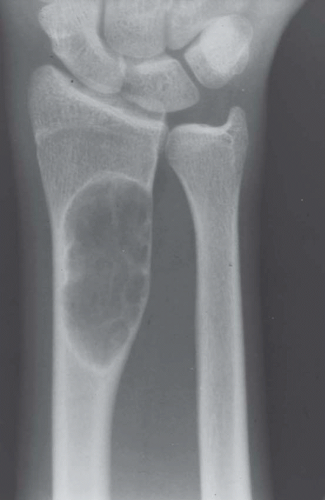
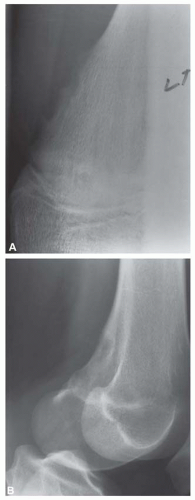
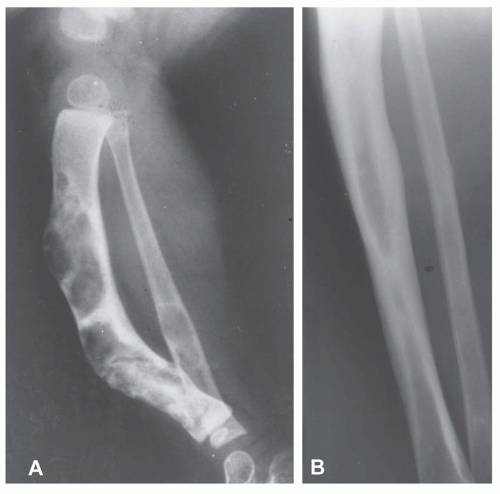
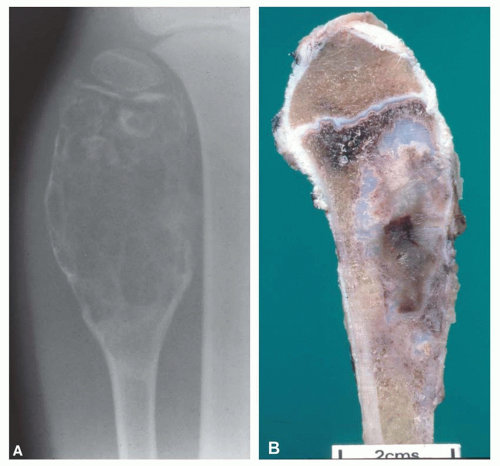
Most metaphyseal fibrous defects have a characteristic radiographic appearance that is virtually diagnostic. When a large tubular bone is affected, the lesion is practically always located eccentrically and often produces some bulging of the cortical outline, which is usually thin over the defect (Figs. 26.14, 26.15, 26.16 and 26.17).
The lucency begins in the metaphysis, near or at the epiphyseal line, and appears to migrate toward the center of the bone as the epiphyseal region grows away from it. The inner boundary of the lesion is demarcated by a thin or prominent scalloped line of sclerosis (Fig. 26.18). Trabeculae frequently appear to traverse a defect and give it a multilocular appearance; however, these trabeculae are nearly always incomplete and the appearance is actually produced by the shadows of corrugations on the inner surface of the cavity that houses the defect. Occasionally, the entire width of the bone may be affected. The radiographic features are so characteristic that usually only chondromyxoid fibroma is in the differential diagnosis.
Gross Pathologic Features
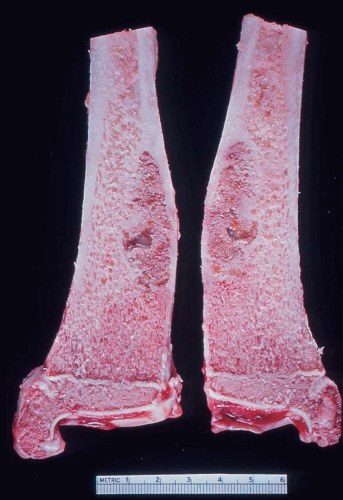
It is unusual to see intact specimens of metaphyseal fibrous defect. Curetted fragments show a granular lesion that is predominantly brown but has foci of yellow discoloration. If the gross specimen is intact, it will have the characteristic lobulated appearance expected from the radiographic features. The lesion attenuates the cortex but does not breach it (Fig. 26.19).
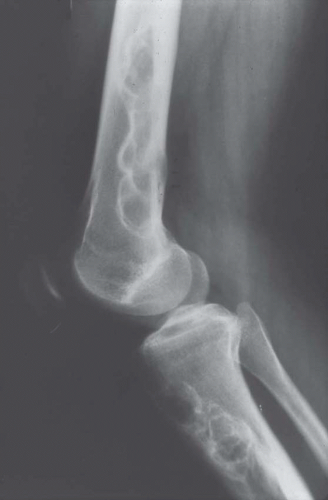 Figure 26.15. Multiple metaphyseal fibrous defects involving the distal femur and proximal tibia. The lesions have a benign radiographic appearance. |
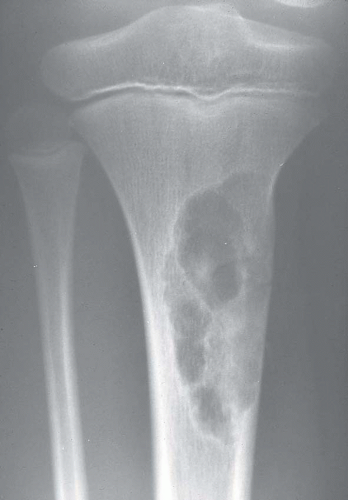 Figure 26.16. Typical radiographic appearance of metaphyseal fibrous defect. The metaphyseal lesion is located eccentrically, is well demarcated, and has a multilocular appearance. |
Histopathologic Features
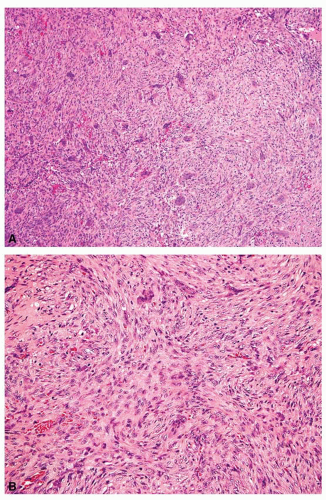
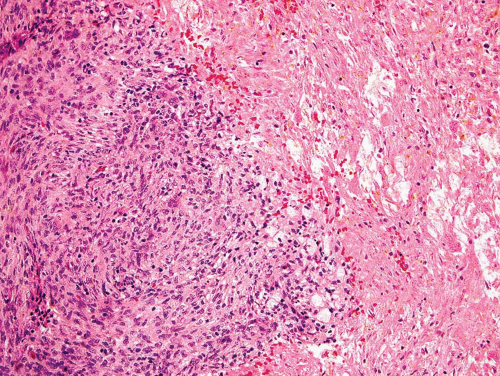
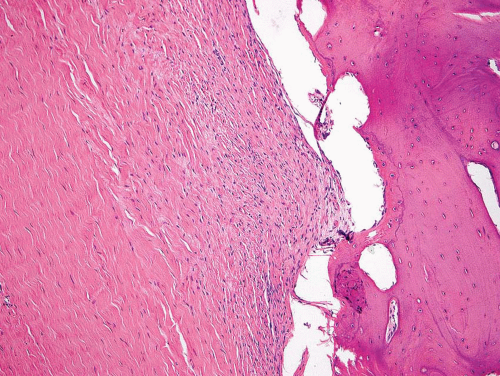
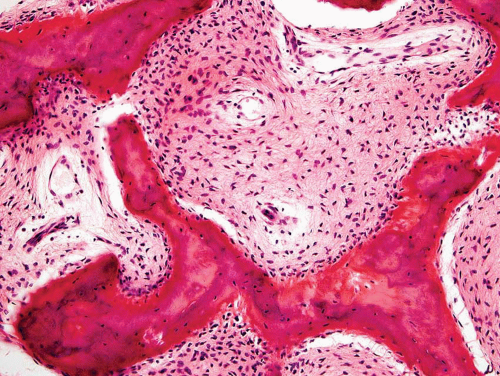
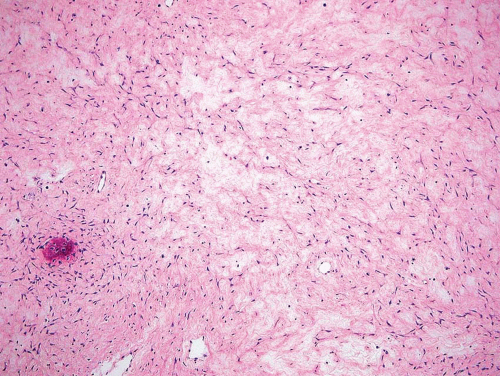
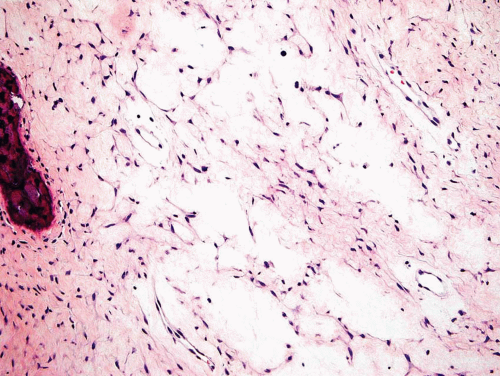
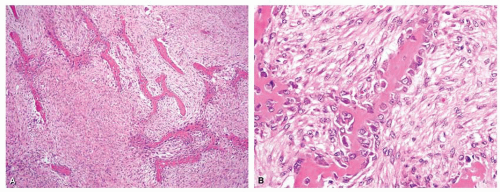
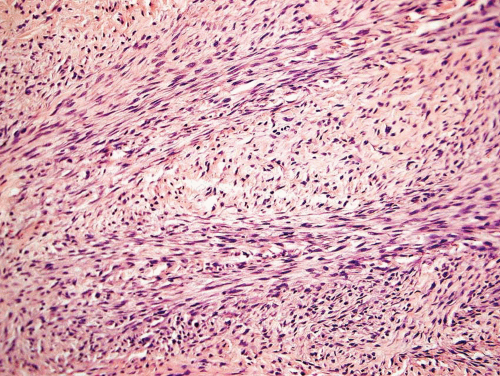
Metaphyseal fibrous defects characteristically show a spindle cell proliferation, with a loose storiform arrangement of the cells (Fig. 26.20). The arrangement of the spindled cells is much less compact than in true fibrohistiocytic neoplasms. The cells are plump but show no hyperchromasia of the nuclei. Mitotic figures may be found (Fig. 26.21). Very characteristically, a yellow to brown pigment, which special stains show to be iron, is present within the spindle cells (Fig. 26.22). Benign giant cells are always found. These are usually in clusters but focally may be very prominent and, out of context, may suggest the diagnosis of a giant cell tumor. Foam cells containing lipid are almost always found in metaphyseal fibrous defect and produce the yellow appearance grossly (Fig. 26.23).
Typically, metaphyseal fibrous defects do not contain bone. However, small foci of reactive new bone formation may be seen, especially in association with a pathologic fracture (Fig. 26.24). Spontaneous necrosis is very unusual unless pathologic fracture has occurred. With pathologic fracture, the lesion may undergo complete infarctlike necrosis (Fig. 26.25).
Because of the presence of giant cells, the lesion may be mistaken for a giant cell tumor. However, the giant cells usually are arranged in clusters, unlike that seen in a true giant cell tumor. The occurrence in the second decade of life and the characteristic location in the metaphysis practically rule out the diagnosis of giant cell tumor. The presence of foam cells, giant cells, and spindle cells in a storiform arrangement may suggest a diagnosis of fibrohistiocytic neoplasm. Indeed, at least some of the so-called fibrous histiocytomas of bone reported in the literature probably represent metaphyseal fibrous defects in unusual locations.
Treatment
If one is confident of the radiographic diagnosis and the structural integrity of the bone is not in question, no treatment is needed and the progress of the lesion
can be followed by repeat radiographs. If the diagnosis is uncertain, diagnosis and therapy can be accomplished with one surgical procedure. The lesion is readily eradicated by conservative surgical means, ordinarily curettage.
can be followed by repeat radiographs. If the diagnosis is uncertain, diagnosis and therapy can be accomplished with one surgical procedure. The lesion is readily eradicated by conservative surgical means, ordinarily curettage.
Prognosis
Most metaphyseal fibrous defects are thought to undergo spontaneous regression. Patients with multiple lesions are at risk for developing multiple pathologic fractures. However, these tend to regress as the skeleton matures.
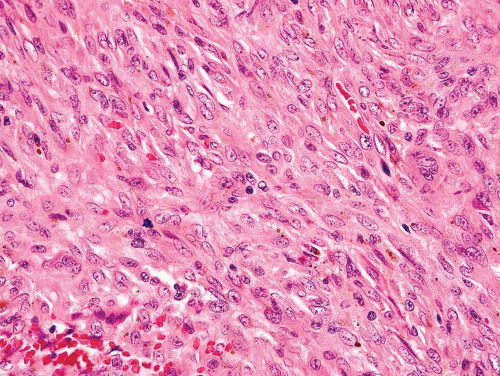 Figure 26.21. Metaphyseal fibrous defect. High-power view shows the plump spindle cells and mitotic activity, which can be worrisome for sarcoma. |
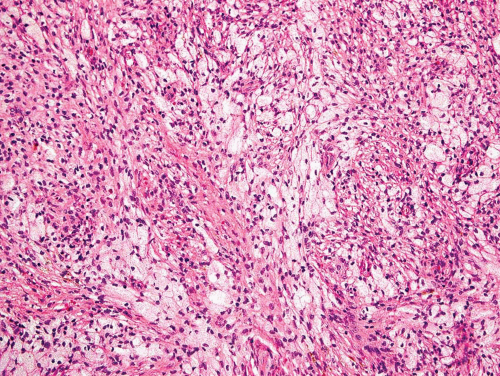 Figure 26.23. Prominent nests of foam cells in fibroblastic stroma within a metaphyseal fibrous defect. |
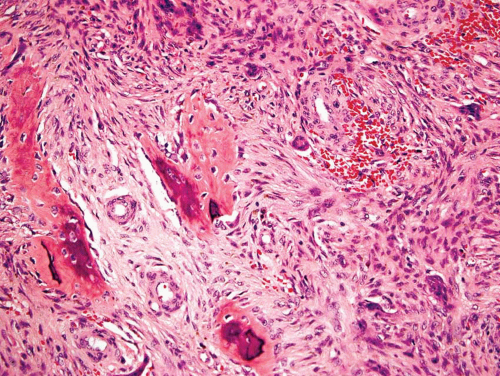 Figure 26.24. Reactive new bone formation within metaphyseal fibrous defect can be seen with or without previous pathologic fracture. |
PERIOSTEAL DESMOID (DISTAL IRREGULARITIES OF THE FEMUR SIMULATING MALIGNANCY, AVULSIVE CORTICAL IRREGULARITY)
Periosteal desmoid refers to a fibrous cortical defect that typically occurs on the posteromedial aspect of the distal femur. They are usually incidental findings on radiographs made for another reason. The cortex shows some irregular destruction, and this may suggest a diagnosis of malignancy. However, the typical location and the small size should lead to the correct diagnosis. If the lesion is removed, the histologic features are those of fibrous replacement of a portion of the cortex. A few benign giant cells may be seen. It is important to be aware of this condition to avoid an unnecessary biopsy (Figs. 26.26 & 26.27).
XANTHOMA OF BONE
The term xanthoma of bone is used when the biopsy specimen shows either a collection of foam cells intermingled with innocuous-appearing spindle cells or cholesterol crystals with foreign body reaction (Fig. 26.28). A lesion may have both features. Forty-three lesions in the Mayo Clinic files were classified as xanthomas.
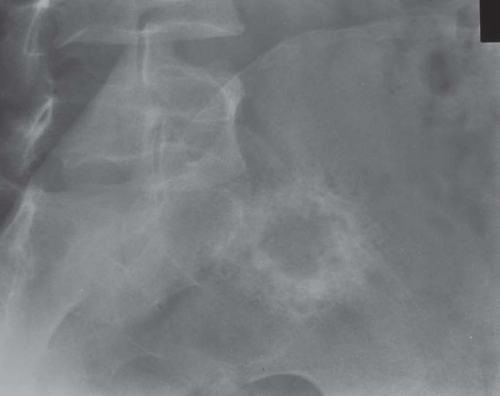
These lesions tend to involve the flat bones such as the skull or innominate bone. They may be painful or discovered as an incidental radiographic finding. Radiographs usually show a well-circumscribed lytic defect with a sclerotic rim (Fig. 26.29). Occasionally, the lesion is completely surrounded by a sclerotic rim. Grossly, the lesion is bright yellow. As mentioned above, microscopically, the lesion shows cholesterol crystals, foam cells, and giant cells.
The differential diagnosis includes many conditions in which foam cells may be found, and the diagnosis of xanthoma is valid only if no other histologic features are found. Fibrous dysplasias, metaphyseal fibrous defects, and giant cell tumors all can contain prominent foam cells. The location and the radiographic features of some of these lesions suggest that they are the end product of an underlying lesion, especially fibrous dysplasia and metaphyseal fibrous defect. It is possible that an occasional giant cell tumor may also undergo regression to become a xanthoma. The most important differential diagnosis involves metastatic clear cell carcinoma, especially hypernephroma. On limited biopsy material, especially with fine-needle aspiration, the foam cells may be mistaken for the cells of a clear cell carcinoma. However, the nuclei of foam cells are usually quite small, and the cytoplasm is abundant. The nuclei of the cells of hypernephroma are usually larger and have nucleoli.
FIBROUS DYSPLASIA
Fibrous dysplasia is probably the result of an aberration in the development of bone. It is characterized by the occurrence of one, a few, or numerous discrete skeletal defects. Yellow or brown patches of cutaneous pigmentation may accompany the bone lesions, especially
in patients with severe disseminated disease. When, in addition to cutaneous pigmentation, such polyostotic disease is accompanied by signs of endocrine abnormality, especially precocious puberty in girls, the condition is commonly called Albright syndrome.
in patients with severe disseminated disease. When, in addition to cutaneous pigmentation, such polyostotic disease is accompanied by signs of endocrine abnormality, especially precocious puberty in girls, the condition is commonly called Albright syndrome.
Fibro-osseous dysplasia is a term that is gaining acceptance for many of the defects involving the base of the skull and the jawbones. Dysplastic lesions at these sites often contain such an abundance of osseous trabeculae intermingled with the fibrous tissue that they are distinctly hard and may cause a dense shadow in the radiograph. Many, if not all, the so-called osteofibromas and fibro-osteomas in these locations are in fact examples of fibro-osseous dysplasia.
Incidence
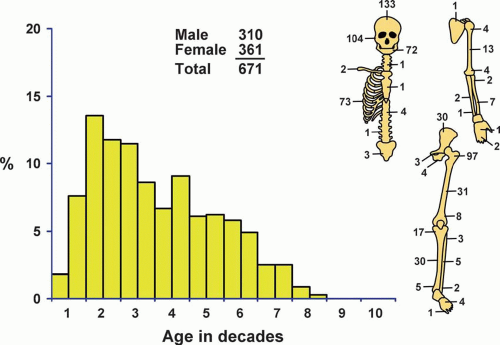
There were a total of 671 fibrous dysplasias in the Mayo Clinic files (Fig. 26.30). This probably does not represent the true incidence because many patients with fibrous dysplasia are asymptomatic.
Sex
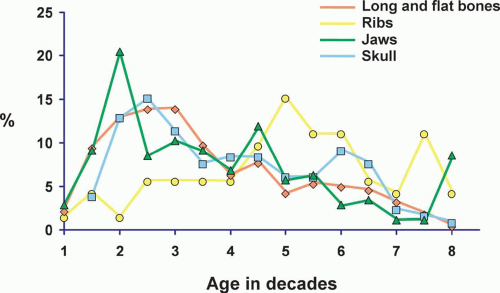
In the overall group of patients with fibrous dysplasia, females predominated slightly. However, there were distinct differences in sex distribution when different sites of involvement with fibrous dysplasia were considered. There was a distinct female predominance in the group of patients with involvement of the jaws and long and flat bones, whereas there was male predominance in the group with rib and skull disease.
Age
The age distribution of patients with fibrous dysplasia involving different sites is given in Figure 26.31. The majority of patients with fibrous dysplasia were in the second and third decades of life. However, patients with fibrous dysplasia of the ribs tend to be older. The explanation of this discrepancy probably is that older patients are more likely to have chest radiography, which shows these incidental findings.
Localization
Patients with fibrous dysplasia were divided into four distinct groups: those with involvement of the jaws, those with involvement of the skull bones, those with involvement of the ribs, and those with involvement of all other bones. The largest single group was the last, and of this group, the proximal femur was by far the most common site. The jawbones accounted for the second largest group, and of these, the maxilla was involved much more commonly than the mandible.
Sixty-four patients had polyostotic fibrous dysplasia. Several patients with involvement of the jawbones had lesions of both the mandible and maxilla; these were not counted as examples of polyostotic fibrous dysplasia.
Symptoms
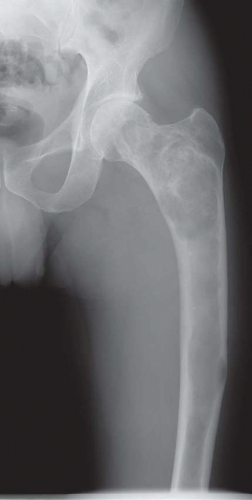
Many lesions of fibrous dysplasia are asymptomatic and are incidental findings on radiographs. This is especially true of the lesions of the ribs. Fibrous dysplasia may present with a pathologic fracture, especially in the femoral neck region. Some examples of fibrous dysplasia may produce swelling, especially in the region of the jaw and the skull. Occasionally, fibrous dysplasia in other locations can also become massive.
Physical Findings
Involvement of the skull and the jawbones with fibrous dysplasia may show deformities. Patients with polyostotic fibrous dysplasia may have characteristic café-au-lait pigmentation of the skin.
Some patients with fibrous dysplasia have associated intramuscular myxomas, as described by Mazabraud. There are four cases of such patients in the Mayo Clinic files. However, fibrous dysplasia was confirmed histologically in only two of these patients; in the other two, radiographs supported the diagnosis of fibrous dysplasia and there was histologic confirmation of soft-tissue myxomas.
Radiographic Features
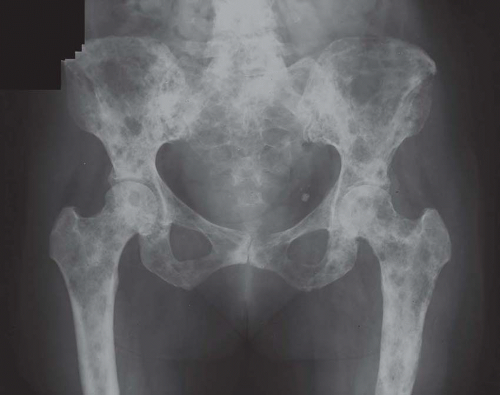
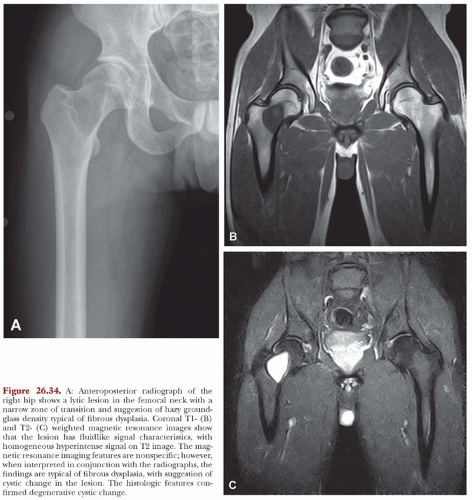
The defects of fibrous dysplasia are usually well-defined zones of rarefaction. The rarefied zone is often surrounded by a narrow rim of relatively sclerotic bone (Figs. 26.32, 26.33, 26.34, 26.35, 26.36 and 26.37). Expansion with thinning of the cortex is especially likely to occur in narrow bones such as the ribs. Occasionally, the lesion produces a large expansile mass that bulges into soft tissues. Lesions with a large osseous component, such as are commonly seen around the base of the skull and maxilla, are likely to be relatively radiopaque. This characteristic is accentuated if the lesion bulges into an air-containing sinus. Some examples of fibrous dysplasia contain large amounts of cartilage, which may be evident on radiographs as ringlike or dotlike calcification. This is especially common in the femoral neck region. Rarely, an example of fibrous dysplasia has a superimposed aneurysmal bone cyst. This may give rise to an aggressive-looking radiographic appearance that suggests the diagnosis of sarcoma.
Gross Pathologic Features
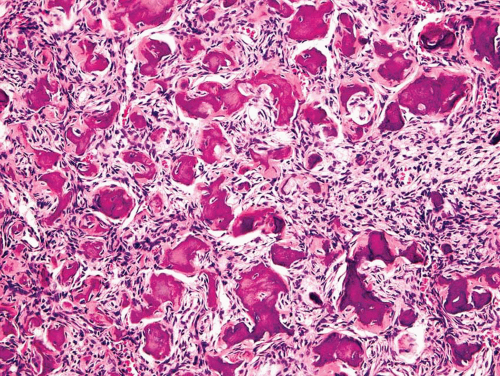
Examination shows considerable variation in the lesions of fibrous dysplasia, but the average lesion is well defined and composed of dense fibrous tissue (Figs. 26.38 & 26.39). Usually embedded in this fibrous tissue are enough small trabeculae of bone to impart a distinctly gritty quality. However, rarely is the ossification sufficient to require decalcification before a section is made. Slight to extensive cyst formation may be present, whereas lesions with pronounced ossification may resemble osteoma. Lesions containing cartilage may have small nodules of well-defined islands of cartilage or the cartilaginous component may dominate the appearance.
Histopathologic Features
The major feature is a proliferation of fibroblasts that produce a dense collagenous matrix. The fibroblasts tend to be plump; they show no cytologic atypia. Mitotic figures are extremely uncommon. In an otherwise typical fibrous dysplasia, one may find large areas without bone production. In these areas, the spindle cells may form a storiform pattern. Characteristic metaplastic bone formation is seen in fibrous dysplasia. Typically, the bony trabeculae show no osteoblastic rimming. However, the presence of osteoblastic rimming does not rule out the possibility of fibrous dysplasia. The bony trabeculae form a woven bone pattern unlike the architecture in mature bone. The bony trabeculae are arranged in a meaningless fashion and may have peculiar shapes characterized as “Chinese characters” (Figs. 26.40, 26.41, 26.42, 26.43, 26.44, 26.45 and 26.46). Occasionally, fibrous dysplasia shows trabecular bone formation, especially in the jawbones. The bony trabeculae may form rounded ossicle-like structures resembling psammoma bodies (Fig. 26.47). This pattern is especially prominent in the base of the skull and may lead to a mistaken diagnosis of meningioma. However, this pattern may also be seen in other locations.
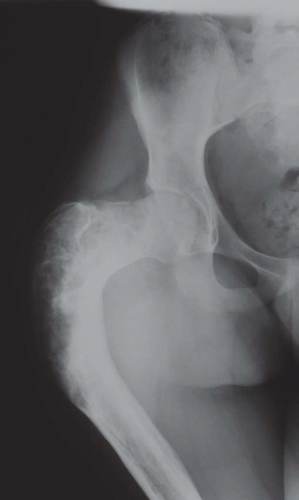 Figure 26.33. Typical appearance of a femur in a patient with polyostotic fibrous dysplasia. The deformity in the proximal femur has been referred to as “shepherd’s crook deformity.” |
Collections of foam cells almost always occur in fibrous dysplasia and may be mistaken for metastatic clear cell carcinoma, especially in limited biopsy samples. Clusters of giant cells are also commonly seen in fibrous dysplasia (Fig. 26.48). Occasionally, one may find large collections of giant cells simulating the appearance of a true giant cell tumor.
Some examples of fibrous dysplasia show marked myxoid change of the matrix. The lesion is extremely hypocellular, and characteristic areas are seen only
at the periphery (Figs. 26.49 & 26.50). Many of the fibromyxomas reported in the literature are undoubtedly examples of myxoid fibrous dysplasia.
at the periphery (Figs. 26.49 & 26.50). Many of the fibromyxomas reported in the literature are undoubtedly examples of myxoid fibrous dysplasia.
Large islands of cartilage may dominate the histologic appearance of fibrous dysplasia. The cartilage forms rounded nodules or, occasionally, plate-like structures that resemble epiphyseal plates (Fig. 26.51). Secondary aneurysmal bone cyst areas may be seen engrafted upon fibrous dysplasia.
Treatment
Treatment should be conservative. The lesions commonly stop growing when the patient reaches puberty. Therapy should be directed at restoring the normal configuration when the skull or jawbones are affected. In large bones, deformity caused by the disease may require correction. Radiation therapy is probably of no value and introduces a hazard of sarcomatous change.
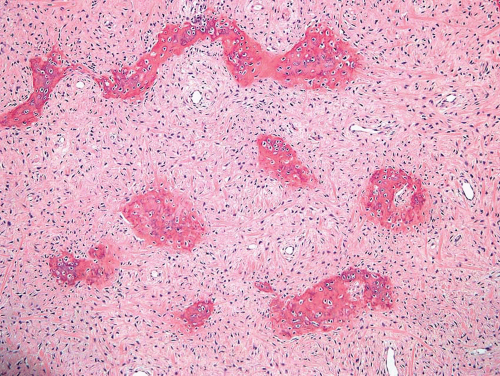 Figure 26.41. Another example of fibrous dysplasia shows the characteristic pattern of woven bone formation and short spindle cells within the stroma. |
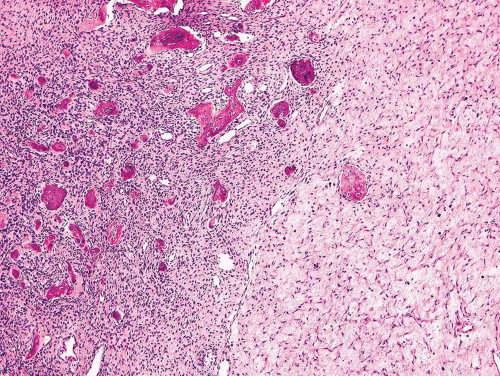 Figure 26.42. Variable stromal cellularity within fibrous dysplasia. Hypocellular spindle cell stroma on the right merges with hypercellular area on the left. |
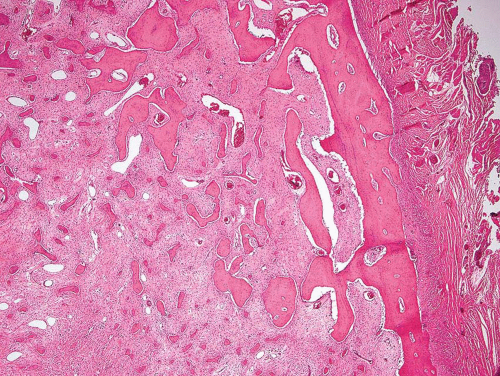 Figure 26.43. Fibrous dysplasia frequently involves the rib, as in this example. The tumor forms an expansile mass that pushes into surrounding soft tissue. |
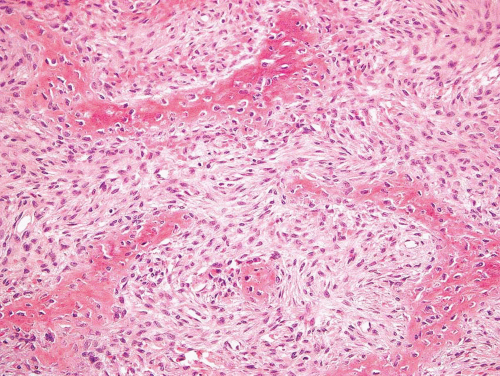 Figure 26.45. In this example of fibrous dysplasia, woven bone appears to be emerging from the fibrous stroma. |
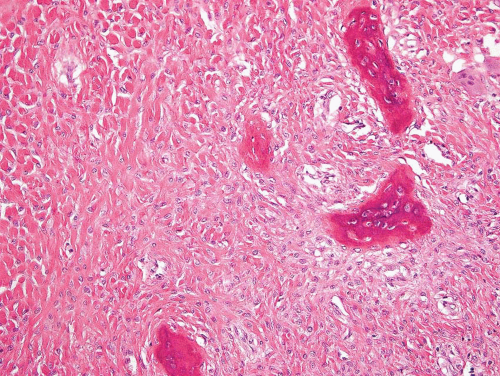 Figure 26.46. Abundant collagen separates stromal cells in this example of fibrous dysplasia involving a rib. |
Prognosis
The prognosis in fibrous dysplasia is good. Deforming lesions of the jawbones or skull may sometimes recur, but these lesions ordinarily respond favorably to additional conservative surgical therapy. Some of the large lesions in weight-bearing bones require curettage and bone grafting for maintenance of function. Patients with extensive disease of the ribs may experience respiratory problems. In our files, one patient with polyostotic fibrous dysplasia of the ribs died of respiratory complications.
SARCOMAlS IN FIBROUS DYSPLASIA
Rarely, malignancies can arise in fibrous dysplasia (Figs. 26.52, 26.53 and 26.54). In 1972, Huvos and coauthors documented, from the files of the Memorial Hospital in New York, 12 examples of sarcomas arising in fibrous dysplasia. Of these patients, only one had previously had radiation treatment. Ruggieri and coauthors reported on 28 cases from the Mayo Clinic files. There were 1,122 examples of fibrous dysplasia in this series, which included cases seen in consultation. Thirteen of the patients had previous radiation therapy; hence, these sarcomas can be considered postradiation.
There are now 19 examples of sarcomas associated with fibrous dysplasia in the Mayo Clinic files. Twelve patients had monostotic fibrous dysplasia, and seven had polyostotic fibrous dysplasia. Of these 19 patients, 12 previously had radiation as part of the treatment of fibrous dysplasia. Seven of the lesions involved the jawbones and two the skull. Twelve of the secondary sarcomas were considered osteosarcoma, four were considered fibrosarcoma, and three were chondrosarcomas. One of the chondrosarcomas was of the clear cell type. The prognosis for these patients was poor. One patient with a lesion of the maxilla was alive without evidence of disease 11.5 years later. Fourteen patients have died of disease at intervals ranging from 4 months to 28 years. No follow-up is available for four patients. It used to be thought that sarcomas arising in fibrous dysplasia are extremely unusual without previous radiation, but it now appears that sarcomas
do arise spontaneously in fibrous dysplasia. However, the incidence is still low (Fig. 26.54).
do arise spontaneously in fibrous dysplasia. However, the incidence is still low (Fig. 26.54).
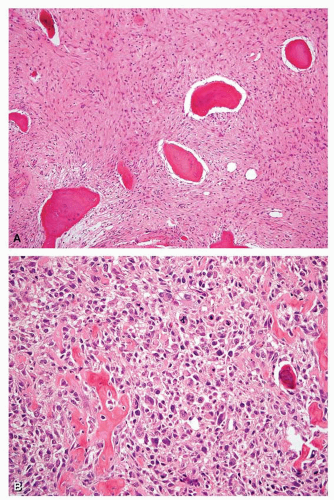 Figure 26.54. Histologic features corresponding to the tumor in Figure 26.53. A: Proximal part of the tibial tumor shows features typical of fibrous dysplasia. B: High-grade osteosarcoma corresponding to the lytic area of the tumor. The patient had not been treated with radiation. He was alive without evidence of disease 14 years after treatment that included surgery and chemotherapy. |
OSTEOFIBROUS DYSPLASIA
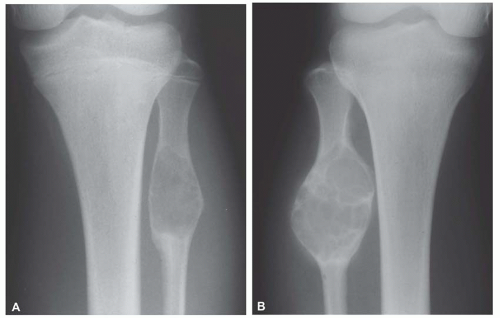
Osteofibrous dysplasia, originally described as ossifying fibroma by Kempson in 1966, has generated much controversy recently. Kempson believed that it was an aggressive lesion, although it was histologically similar to fibrous dysplasia. Campanacci thought that the lesion was benign and underwent spontaneous regression. He preferred the term osteofibrous dysplasia (Figs. 26.55, 26.56 and 26.57).
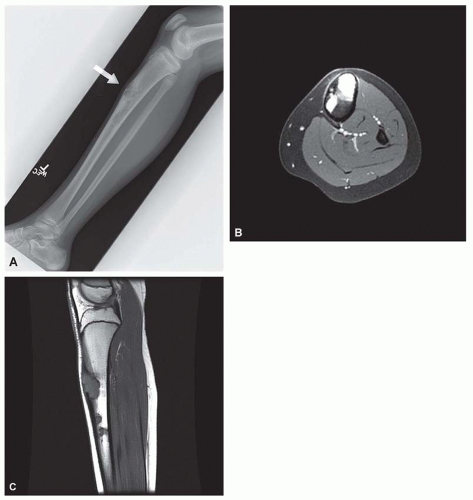
Osteofibrous dysplasia has several peculiarities, the most pronounced being its tendency to involve the tibia and, less often, the fibula. It also tends to involve the cortex of the bone. Although it is predominantly a disease of patients in the first two decades of life, even adults with the disease have been described. Radiographs show multiple lucencies involving the anterior cortex of the tibia, with intervening sclerosis (Figs. 26.55 & 26.56). This radiographic appearance is very similar to that of adamantinoma of the
tibia. Several studies have shown the presence of keratin-positive cells in osteofibrous dysplasia. This has led to the suggestion that osteofibrous dysplasia and adamantinoma may be related conditions. One study postulated that osteofibrous dysplasia is a regressed form of adamantinoma. The other possibility suggested is that osteofibrous dysplasia may progress to adamantinoma. In studies involving osteofibrous dysplasia of the long bones, no progression of this condition into adamantinoma was noted.
tibia. Several studies have shown the presence of keratin-positive cells in osteofibrous dysplasia. This has led to the suggestion that osteofibrous dysplasia and adamantinoma may be related conditions. One study postulated that osteofibrous dysplasia is a regressed form of adamantinoma. The other possibility suggested is that osteofibrous dysplasia may progress to adamantinoma. In studies involving osteofibrous dysplasia of the long bones, no progression of this condition into adamantinoma was noted.
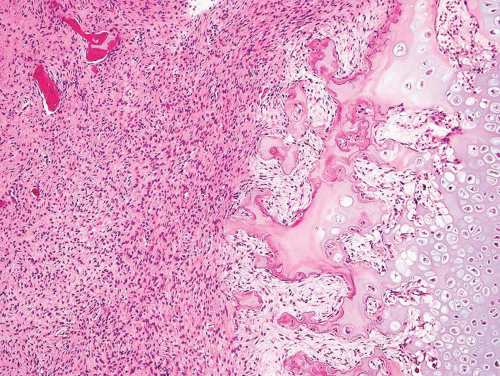
As noted above, osteofibrous dysplasia involves the cortex, whereas classic fibrous dysplasia is a disease of the medullary bone. The lesion is composed of a spindle cell proliferation that may have a storiform pattern. The bony trabeculae show prominent osteoblastic rimming, in contrast to that seen in fibrous dysplasia (Fig. 26.57). The lesion also tends to mature toward the periphery and seems to merge with cortical bone, giving rise to a zonation phenomenon.
The relationship between osteofibrous dysplasia and adamantinoma, if any, is still unclear. Osteofibrous dysplasia probably is a form of fibrous dysplasia that is confined to the tibia and fibula and has a tendency to involve the cortex.
FIBROCARTILAGINOUS MESENCHYMOMA
Fibrocartilaginous mesenchymoma was first described by Dahlin and coauthors in 1984. These authors described five cases of an entity that had previously been classified with fibrous dysplasia. Because of a tendency for local recurrence, the term fibrocartilaginous mesenchymoma with low-grade malignancy was used. Bulychova and coauthors published 12 cases, including those of 1984. Longer term follow-up suggested that although the lesion may recur, there has been no instance of malignant behavior. Hence, the more noncommittal term fibrocartilaginous mesenchymoma of bone was thought to be more appropriate.
Fibrocartilaginous mesenchymoma is one of the rarest lesions of bone. The Mayo Clinic files contain only one example, a case involving the pubis in a 19-year-old man. The lesion does affect young people. The proximal fibula is one of the sites of predilection.
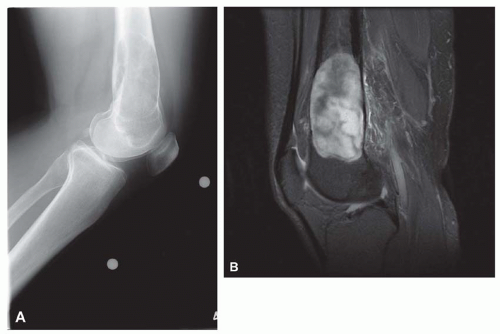
On radiographs, the lesions tend to involve the metaphyseal portion of the bone abutting on the growth plate. The lesions are predominantly lucent but have some mineralization, suggesting a cartilaginous component (Fig. 26.58).
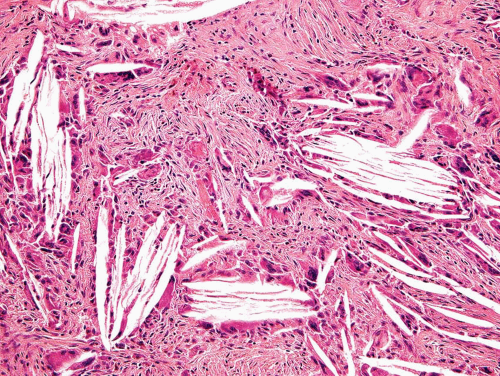
Microscopically, the lesion shows a combination of cartilage, bone formation, and spindle cell proliferation. The cartilage has a very characteristic arrangement in plates with enchondral bone formation, simulating the appearance of epiphyseal plates (Fig. 26.59). A densely cellular spindle cell proliferation is found between well-formed bony trabeculae. The spindle cells are elongated and show little collagen production, unlike the appearance in fibrous dysplasia (Fig. 26.60).
Many of the lesions have not been adequately treated surgically. Although local recurrences were not uncommon, these were usually managed with repeat excision. No patient in the series described by Bulychova and coauthors has died of disease.
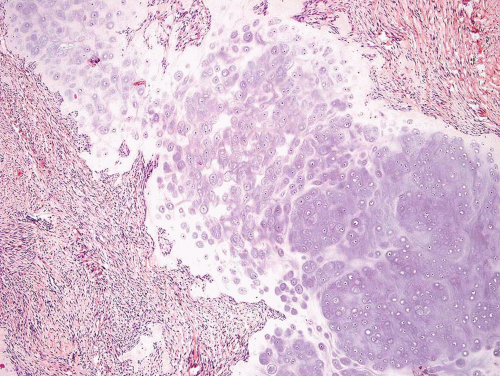 Figure 26.59. Low-power view of fibrocartilaginous mesenchymoma. The field is dominated by a plate of cartilage. Occasionally, the cartilage has features similar to those of epiphyseal cartilage. |
MYOFIBROMA (INFANTILE MYOFIBROMATOSIS, CONGENITAL FIBROMATOSIS)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree