Chapter 125 Complications of Total Knee Arthroplasty
General Complications
Prevention of medical complications begins with the preoperative evaluation. Prior to total knee arthroplasty, the patient should be evaluated by the primary care physician for preoperative medical clearance. The orthopedic surgeon is also responsible for reviewing the medical history and should note the comorbidities that must be addressed to minimize the chance of complication. Memtsoudis et al151 has recently reported on in-hospital complications, including mortality, following unilateral, bilateral, and revision total knee arthroplasty. Over 4 million discharges were evaluated with regard to patient demographics, comorbidities, and in-hospital stay. Complications and mortality of each procedure were compared. In this series, in-hospital mortality was highest for patients who had bilateral total knee arthroplasty (0.5%) whereas the lowest mortality rate was associated with unicompartmental TKA (0.3%). It was interesting to note that revision total knee arthroplasties had a similarly low mortality rate of 0.3%. The overall complication rate during hospitalization was highest for bilateral total knee arthroplasties, 12.2%, but the rate for in-hospital complications following unicompartmental TKA was still 8.2%.
A patient’s medical history should be reviewed carefully if bilateral TKA is contemplated. Although several studies have demonstrated success in bilateral procedures, numerous studies have demonstrated the higher risk of complication.28,50 Younger patients in good health seem to tolerate a bilateral procedure with little increased risk. However, older or obese patients, and those with an extensive medical or cardiac history, should be discouraged from considering a bilateral procedure. If a bilateral procedure is performed, postoperative monitoring should be considered.
Systemic Complications
Thromboembolism
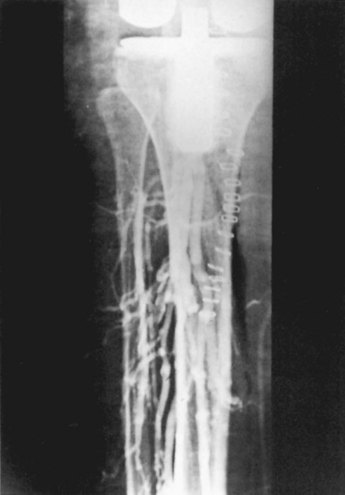
DVT occurs in approximately 50% of unilateral cases and in 75% of bilateral cases when no prophylaxis is used. Although DVT occurs mainly in the calf veins (Fig. 125-1), life-threatening emboli do not arise from this region. In contrast to the situation after total hip arthroplasty (THA), isolated proximal vein thrombosis does not seem to occur after knee surgery, despite the possible trauma of a pneumatic tourniquet.
Some have argued that distal thrombosis can be ignored, provided that the patient convalesces normally and is not confined to bed for a lengthy period. We believe that this view is too optimistic, although we concede that the risk of a fatal thromboembolus is small and is lower than that after THA. In a prospective review of 527 TKAs in 499 patients, Khaw and colleagues,118 using no prophylaxis other than antithrombotic stockings and relatively early (48 hours) mobilization, found only one death in 3 months from pulmonary embolism (0.19%). This patient had bilateral TKA and had a myocardial infarction 1 day postoperatively, with the subsequent pulmonary embolus occurring 22 days postoperatively. Seven other patients (1.3%) developed symptomatic pulmonary embolism and were treated with anticoagulation, without sequelae. This low incidence of fatal pulmonary embolism has been supported in other studies by Stulberg and associates (0%),214 Stringer and coworkers (0%),213 Khaw and colleagues (0.2%),118 and Ansari and associates (0.4%).4 Fatal emboli do occur, however, and three patients died as a result of emboli in the first 400 arthroplasties performed at the Hospital for Special Surgery. No specific prophylaxis was used at that time; the cause of death was confirmed by autopsy in all cases. In addition, we believe that the incidence of fatal pulmonary emboli is underestimated. A certain proportion of calf clots propagate proximally to form the more dangerous clots in the popliteal and femoral veins. This process takes time, however, and the major risk period for a fatal pulmonary embolism after TKA may be in weeks 3 and 4 postsurgery; this is in contrast to what occurs after THA, in which a clot in the proximal veins may be found in 20% of cases after the first week. Because most TKA patients now are discharged after hospitalization of 2 to 3 days, sudden death at home in a patient having no clinical evidence of vein thrombosis may be attributed to myocardial infarction.
Calf clots may not in themselves be important, but should be regarded as a harbinger of more proximal clotting. Haas and coworkers85 have studied 1329 patients with 1697 TKAs. Thrombosis was found in 808 patients (61%); 53% had thrombosis of the calf vein and 8% had thrombosis of the proximal veins. The lung scans of 60 patients (4.5%) were positive, and symptomatic pulmonary emboli occurred in 14 patients (1.1%). All these patients received aspirin as prophylaxis. Venography was performed between postoperative days 4 and 6. A perfusion lung scan obtained on postoperative day 5 to 7 was compared with a preoperative baseline perfusion lung scan. Thrombosis of the calf vein was treated with warfarin (Coumadin); the dosage was adjusted to maintain a prothrombin time at approximately 1.5 times control. Only patients with symptomatic proximal thrombi or symptomatic pulmonary emboli were fully anticoagulated with intravenous heparin. Although the natural history of thromboembolic disease was altered by prophylaxis and treatment, 6.5% of patients with calf thrombi had a positive lung scan and 1.6% had symptomatic pulmonary emboli. This finding was compared with patients who had no venographic evidence of deep vein thrombosis (DVT), of whom 1.9% had a positive lung scan and 0.2% had symptomatic pulmonary emboli. Statistical significances for the difference in lung scan and pulmonary embolus results were P = .001 and P = .034, respectively. Patients with calf or proximal thrombi were found to have similar rates of positive lung scans and symptomatic pulmonary emboli.
The risk of symptomatic and fatal pulmonary embolism is of greatest concern. Without prophylaxis, the rates of pulmonary embolism and fatal pulmonary embolism range from 1% to 28% and 0.1% to 2.0%, respectively.74 From six randomized studies comparing low-molecular-weight heparin (LMWH) with warfarin, the total rate of DVT was 33% for patients who received LMWH compared with 48% for patients who received warfarin. Similarly, the rate of proximal DVT was 7.1% and 10.4% for patients who received LMWH and warfarin, respectively. Barrett and colleagues11 have reviewed 122,385 U.S. Medicare enrollees who had a TKA in 2000. Pulmonary embolism developed in the first 3 months in 0.81% of patients who had a unilateral TKA compared with 1.44% of patients who had undergone a simultaneous bilateral TKA.
Protocol for Patients With Documented Thromboembolic Disease
Positive Venography or Ultrasound
Patients with calf, popliteal, or femoral thrombi are treated with warfarin for 6 weeks. Asymptomatic pulmonary emboli are treated in the same manner. Symptomatic proximal thrombi and symptomatic pulmonary emboli are treated with heparin until the effect of the warfarin is established.225 Based on the discretion of the individual medical consultant, heparin treatment intravenously may be continued for 1 week. Intravenous heparin carries an extreme risk of local bleeding complications and, in our opinion, should be used only in potentially life-threatening situations. This policy sometimes may cause conflict among medical advisors, who often may wish to use heparin in less threatening circumstances. Orthopedic surgeons should preemptively discuss and establish a policy and guidelines within their own institution on which surgeons and medical consultants can agree.
Greenfield Filter
A Greenfield filter should be considered when pulmonary embolism occurs despite therapeutic warfarin prophylaxis, when warfarin is contraindicated in a high-risk patient, or when complications develop as a result of anticoagulation. Vaughn and associates222 have reported on its use in 66 patients and found the technique to be safe, easy, and effective. They inserted the filter preoperatively in 42 patients who were considered at high risk for pulmonary embolus (group I) and postoperatively in 24 patients (group II). The preferred site of insertion was by way of the right internal jugular vein, and a vascular surgeon carried out the implantations. One patient in group II died of a massive pulmonary embolism 3 days after implantation. At follow-up, none of the remaining patients experienced migration of the filter, and there was no evidence of postphlebitic syndrome or chronic symptomatic edema of the lower extremity.
Fat Embolism Syndrome
The diagnosis of fat embolism can be elusive,20,32,56,64,160 and we suspect that the condition often may pass unrecognized as the cause of transient confusional states after surgery. The syndrome results from the embolization of fat and other debris from the femur or tibia that travels mostly to the lungs. The initial effects in the lungs are mechanical, with an increase in perfusion pressure, engorgement of the vessels in the lungs, and secondary right-sided heart strain. In the presence of hypovolemic shock, the patient may die from acute right-sided heart failure. The delayed effects of fat embolism occur after 48 to 72 hours because of the chemical effects of fat. The pulmonary tissue secretes lipase, which hydrolyzes fat into free fatty acids and glycerol. These free fatty acids increase capillary permeability, cause destruction of alveolar architecture, and damage lung surfactant. The end result of all these changes is hypoxia.183
Clinical findings of fat embolism syndrome include tachypnea, dyspnea, profuse tracheobronchial secretions, apprehension, anxiety, delirium, confusion, unconsciousness, and petechial hemorrhage. Laboratory findings are hypoxemia on arterial blood gas testing and thrombocytopenia lower than 150,000 mm3. Treatment is supportive. Mechanical ventilation may be required in advanced cases. Corticosteroids may be beneficial in diminishing the inflammatory response from the chemical effects of fat emboli.174
Monto and coworkers,160 in a review of the literature, reported 19 cases with 9 deaths. Of the 19 cases, 15 were associated with long-stem cemented prostheses, such as the Guepar hinge. Four cases were associated with total condylar arthroplasty. One case was associated with intramedullary instrumentation. Fahmy and colleagues64 have shown human intramedullary (IM) femoral canal pressures of 500 to 1000 mm Hg generated by using standard alignment rod techniques. Venting the canal did not lower the canal pressure significantly. IM pressures were maintained within normal limits only by overdrilling the femoral canal and gently placing the guide rod. Copious irrigation of the IM canal with pulsatile lavage, suction of marrow contents after irrigation, and use of fluted rods to assist the egress of bone marrow elements may reduce the intramedullary pressure. The use of a pneumatic tourniquet does not protect against fat embolism and, with the popularity of IM guidance systems for femoral and tibial components, an increase in the incidence of fat embolism syndrome may be expected. The surgeon should consider avoiding routine instrumentation of the IM canals of the femur and tibia in bilateral cases.
Young-Hood and Kim and colleagues121 have compared the prevalence of fat embolism after TKA in 160 patients (210 knees) with navigation and 160 patients (210 knees) without navigation. They found no significant difference in the intraoperative and postoperative hemodynamic values between the groups. A higher prevalence of fat embolism was seen (60% vs. 41%) in patients with higher triglyceride levels. However, in contrast, Kalairajah and colleagues113 have demonstrated a significant reduction in the number of cranial fat emboli in a group treated with computer-assisted TKA compared with a group treated with TKA using standard IM instrumentation.
Local Complications
Wound Drainage and Delayed Wound Healing
Preoperative Considerations
General Overview
The knee has a thin, overlying soft tissue envelope that must be protective, well vascularized, and supple enough to allow for the large degrees of stretch and shear required for a functional range of motion (ROM). Although most TKAs can be performed with standard protocols, an understanding of when to apply specific soft tissue management principles is required. Preoperative evaluation for TKA should include not only a complete history and physical examination, including radiographic and clinical assessment of the degree of deformity and joint space narrowing, but also a thorough history and evaluation of the skin. Systemic concerns include vascular compromise, obesity, malnutrition, prolonged corticosteroid or nonsteroidal anti-inflammatory drug use, diabetes mellitus, an immunocompromised state, and a history of smoking.* Local factors that affect wound healing include the inability to incorporate a previous incision into the planned incision, a small skin bridge between the previous incision and the planned incision, local radiation or burns, and dense or adherent scar tissue. Other local factors may play a role as well. The correction of severe deformity may make subsequent closure difficult. Special caution should be used in patients with severe varus and rotational deformity because, as the deformity is corrected, there may not be enough skin to close the inferior aspect of the wound over the subcutaneous surface of tibia. Prior trauma may also play a role because of previously placed skin incisions, significant scarring, and loss of skin mobility.
Planning the Skin Incision
Understanding of the local anatomy and blood supply is also necessary. Terminal branches of the peripatellar anastomotic ring of arteries are responsible for most of the blood supply to the anterior skin and subcutaneous tissues. This occurs through a subdermal plexus supplied by arterioles in the subcutaneous fascia. Thus, flap formation over the anterior aspect of the knee must be limited and performed deep to the subcutaneous fascia. A midline skin incision is optimal and should be used whenever possible. This approach reduces the dimensions of the lateral skin flap at which lower skin oxygen tension is noted. Previous longitudinal incisions can be used safely. Some degree of modification is often required to incorporate previous paramedian incisions. If multiple parallel longitudinal incisions exist, the most lateral incision is chosen, because the predominant blood supply enters medially. Johnson108 has shown a reduction in oxygenation of the skin in the lateral region after skin incisions about the knee by the measurement of transcutaneous oxygen. Clarke and associates37 also have described decreased oxygen tension in the incisional skin margins when using tourniquets. This hypoxia increased with tourniquet tightness. The recommended pressure is 125 mm Hg above the mean blood pressure.
Postoperative Considerations
Local Care
Local care measures begin with frequent dressing changes, elevation of the extremity, and limiting mobilization, including ROM activities. When a wound is identified to be at risk in the early postoperative period, we routinely discontinue continuous passive motion, apply a compressive dressing, place the knee in an immobilizer, and institute physical therapy. When superficial epidermal loss occurs in an area smaller than 2 to 3 cm2, several modified dressing protocols can be instituted to protect the underlying tissues and allow for secondary healing (Fig. 125-2). These include antibacterial ointments or gels and enzymatic débriding agents. During this phase, we may consider temporary discontinuation of the anticoagulant agent, because a hematoma related to overaggressive anticoagulation can be devastating at this stage of healing. The use of mechanical devices for DVT prophylaxis should be considered until the wound stabilizes.
Irrigation and Débridement
Burnett and coworkers30 have reported that the rates of surgical site complications necessitating readmission, irrigation and débridement of hematoma and the wound, or prolonged hospitalization for wound drainage are 4.7%, 3.4%, and 5.1%, respectively. Wound drainage occurred for 4 to 7 days after 9.3% of the procedures and for more than 7 days after 9.3% of the procedures, with more than 7 days of drainage being highly predictive of readmission and wound reoperation.
Galat and colleagues,72 in their study of 17,784 primary TKAs, have reported that the rate of early return to surgery for wound complication is 0.33%. They found that the 2-year cumulative probabilities of major subsequent surgery (component resection, muscle flap coverage, or amputation) and deep infection were 5.3% and 6.0%, respectively, in the knees with early surgical treatment of wound complications as compared with 0.6% and 0.8%, respectively, for knees without early surgical intervention. The overall prosthesis salvage rate was 98%, but obviously results are inferior compared with those of patients without early wound complication.
Hematoma
Wound drainage is of particular concern. Weiss and Krackow228 have described an increased rate of knee infection with prolonged wound drainage. Patel and associates173 have evaluated factors that lead to prolonged drainage in the postoperative period. The authors have demonstrated that an increase in the initial postoperative drainage in the recovery room was most indicative for further drainage on days 2, 3, 4, and 5. In other words, better control in the immediate postoperative period, with less postoperative recovery room drainage, led to less wound drainage on subsequent hospitalization days. Limiting blood loss in the recovery room begins in the operating room (OR). Plugging of the intramedullary canal can help decrease initial blood loss as can cauterization of known sources of bleeding, such as genicular vessels and the posterior capsule49 (Fig. 125-3). Intraoperative tourniquet deflation has not been shown to decrease blood loss; in fact, some studies have shown an increase in blood loss when this is performed.182 The appropriate timing of tourniquet release remains debated, even among surgeons at our institution. The improved designs of TKA and improved surgical techniques have led to improved patellar tracking, resulting in less wound drainage when the lateral release is avoided. At our institution, we use a multimodal approach to minimizing intraoperative bleeding, including a smaller quadriceps-sparing incision, meticulous surgical technique, lidocaine and epinephrine injection, bipolar sealer device, and a topical fibrin spray.
When postoperative hematoma occurs, a period of immobilization and observation is permissible, and many hematomas subside spontaneously. If bleeding continues, as evidenced by tense and painful swelling, the knee must be reopened and the source of the bleeding identified (an argument for intraoperative tourniquet release). Probing and squeezing the wound to evacuate a hematoma are not recommended because this could lead to retrograde contamination. Indications for surgical evacuation of a hematoma include skin compromise, wound dehiscence, impending skin necrosis, leakage through the incision, persistent sanguineous drainage, and pain. Galat and associates71 have studied 42 TKA patients matched with 42 control subjects. They found that the rate of return to surgery within 30 days for evacuation of a postoperative hematoma was 0.24%. The 2-year rates of a subsequent major operation or deep periprosthetic infection in the study group were 12.3% and 13.6%, respectively and 0.9% and 1.4%, respectively, in the control group.
Vascular Complications
Arterial complications are rare,130,185 and the preoperative absence of peripheral pulses has not been regarded as a contraindication to surgery, provided that the capillary circulation was adequate. Vascular complication has been reported in 0.03% to 0.2% of cases.47 Injury to the popliteal artery can occur during resection of the proximal tibia or posterior femoral condyles and while releasing the posterior capsule or posterior collateral ligament (PCL). Failure to remove protruding cement from the back of the tibia can also cause direct damage or thermal injury.
Da Silva and Sobel51 have reported on 19 patients with TKA-related popliteal artery injury. Of these, 84% (16/19) of patients had full recoveries. Limb loss occurred in 2 of 19 patients (10.5%). Parvizi and coworkers172 collected data on 13,517 patients undergoing total joint arthroplasty and reported 16 vascular injuries (0.1%). Eleven injuries occurred after TKA and five after THA. Of the 16 patients, 8 (50%) had launched a legal suit against the operating surgeon. Abularrage and colleagues,1 in their prospective study of 41,633 patients, evaluated the predictors of lower extremity arterial injury after TKA or THA. They found that the revision procedures and African American race were statistically significant predictors of arterial injury.
Numerous studies have reported arteriovenous fistula formation, arterial aneurysm, and pseudoaneurysm following TKA. A possible reason may be an unrecognized injury to the perigenicular vessels, leading to hemarthrosis. Most present within 6 months of surgery but some may be delayed. In a case reported by Sharma and associates,210 three large hemorrhagic effusions occurred 4 weeks after total knee replacement (TKR). An arteriogram revealed a pseudoaneurysm filling from the inferior medial genicular artery. Ibrahim and coworkers102 have reported two cases of pseudoaneurysm after TKR, presenting after 5 days and 1 month, respectively. The first patient was successfully embolized and the second patient was treated by injecting a solution of thrombin into the pseudoaneurysm to produce immediate thrombosis without impairing flow across the vessel. Sandoval and colleagues202 have reported a case of popliteal pseudoaneurysm following TKA. Open vascular surgery with resection of the pseudoaneurysm and end to end bypass of contralateral saphenous vein graft was successfully performed. Their possible explanation was perforation of the anterior wall of the popliteal artery during the TKA. Haddad and associates86 have reported a case of recurrent hemarthrosis following TKR after a previous dome tibial osteotomy for medial compartment osteoarthritis. Recurrent hemarthrosis in this case was secondary to arteriovenous (AV) fistula of the peroneal artery at the site of the previous fibular osteotomy. Thomas and coworkers218 have reported an iatrogenic popliteal AV fistula 3 years after TKA. This was successfully treated by resection of the fistula and direct repair of the artery and vein.
Acute arterial occlusion can be seen after TKA. Rates in the literature have been reported from 0.03% to 0.17%.125 Possible causes include manual manipulation to reduce a flexion contracture58,147 and the use of tourniquet. Pressure on a preexisting atheromatous plaque may cause release of the plaque, which is then lodged into a more distal artery. Gregory and colleagues81 have reported a case of an 81-year-old patient with acute arterial occlusion 9 days after TKA. This was successfully treated by urgent surgical embolectomy.
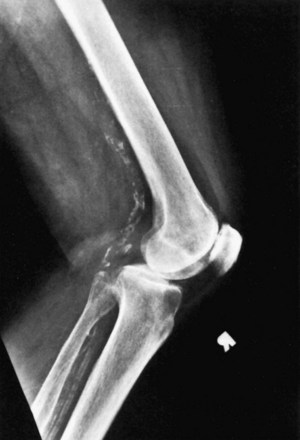
It is important to determine and document the presence of peripheral vascular disease, arterial calcifications, and popliteal aneurysm preoperatively (Fig. 125-4). Absolute vascular contraindications for performing a TKA include the presence of verified vascular claudication with minimal or no activity, active skin ulcerations secondary to arterial insufficiency or venous stasis, and ischemia or frank necrosis in the toes. Others have cautioned against the use of a tourniquet after previous bypass surgery.
Nerve Palsy and Neurologic Complications
Anatomy of the Peroneal Nerve
The superficial peroneal nerve passes distally between the peronei and extensor digitorum longus. Motor branches are given off to the peroneus longus and peroneus brevis. In the lower third of the leg, the nerve branches into the medial and intermediate dorsal cutaneous nerves. These terminal branches complete the sensory innervation of the feet. Bruzzone and associates26 identified anatomic landmarks after 20 cadaveric knee dissections and defined a danger zone and a safe zone to avoid common peroneal nerve injury when performing the inside-out release technique of the posterior-lateral corner during TKA. During the release of the posterior-lateral capsule, the nerve was identified to be at risk in the triangle defined by the popliteus tendon, tibial cut surface, and most posterior fibers of the iliotibial band (danger zone), but not during “pie-crusting” of the iliotibial band (safe zone). They also defined the average distance from the nerve to the posterior-lateral corner of the tibia and to the posterior border of the iliotibial band as 13.5 and 35.8 mm, respectively.
Postoperative Clinical Findings
Peroneal nerve palsy is an infrequent but worrisome complication after TKA. The incidence is higher in revision cases than primary TKA. The prevalence of peroneal nerve palsy has been reported many times in the literature. Mont and coworkers157 have reviewed the literature and found the cumulative prevalence to be 0.58% (74 of 12,784). Asp and Rand6 reported an incidence of 0.3% in 8754 TKAs performed at the Mayo Clinic. Yacub and colleagues,240 in their retrospective study of 14,979 patients, reported a 0.01% incidence of nerve injury after TKA. They noted a 10-fold difference in nerve injury rates between diabetic and nondiabetic patients, 0.11% versus 0.01%, respectively.
Numerous causes for nerve palsy have been described, but they are most commonly associated with the correction of severe flexion and valgus deformity or a combination of the two. The following factors contribute to the development of peroneal nerve palsy: (1) stretching of the nerve in valgus and flexion contractures; (2) fascial compression of the nerve and its vascular supply; (3) direct pressure from the postoperative dressing; and (4) epidural anesthesia. In rare idiopathic cases, none of the mechanisms listed seem to apply. Factors that have not been found to be associated with peroneal nerve palsy include age, gender, type of arthritis, and duration of tourniquet. Idusuyi and Morrey103 performed 10,321 TKAs from 1979 through 1992 at the Mayo Clinic and reported 32 postoperative peroneal nerve palsies. The factors associated with peroneal nerve palsies in this series were epidural anesthesia for postoperative pain control, previous laminectomy, and preoperative valgus deformity. The relative risk for patients who had previous proximal tibial osteotomy was doubled but was not statistically significant.
Postoperative dressings that are too tight or improperly padded can cause direct pressure on the peroneal nerve, causing nerve palsy. Beller and associates,15 in their study, described a double crush syndrome, which includes unrecognized pressure on the peroneal nerve caused by continuous epidural anesthesia and an axonal lesion from the pressure of the pneumatic tourniquet. To prevent a peroneal lesion after TKA while using continuous epidural anaesthesia, they recommended limiting the pneumatic tourniquet pressure to 320 mm Hg and ensuring pressure-free positioning of the operated leg.
Asp and Rand6 performed 8998 arthroplasties and reported 26 nerve palsies. They noted that complete recovery was more likely when the palsy was initially incomplete. Dressing removal and flexion of the knee on diagnosis did not always help. The time of presentation of the problem varied from discovery in the recovery room to postoperative day 6. The motor fibers of the tibialis anterior and extensor hallucis longus muscles were affected in all cases; a sensory deficit was noticed in 20 patients (87%). The peroneus longus muscle was affected in nine patients (39%). Electromyographic evaluation showed a diffuse motor neuropathy in the mild cases and denervation potentials in muscles supplied by the deep branch of the common peroneal nerve in the more severely involved cases. The treatment rendered on discovery of these findings varied according to the discretion of the surgeon involved. The most frequent therapeutic measure was to loosen the Robert Jones dressing and place the knee in a more flexed position. In two cases, this maneuver brought immediate improvement of motor and sensory deficits. The time interval between discovery and the beginning of the return of function ranged from immediately in the two cases mentioned to 6 months. Motor improvement occurred first, with sensory return lagging behind.
Omeroglu and coworkers166 have reported the case of 46-year-old rheumatoid patient with bilateral peroneal nerve palsy on the second postoperative day after simultaneous bilateral TKA. Electromyographic studies revealed bilateral axonotmesis. Complete motor recovery was seen on both sides within 6 months, although sensory deficit was present on one side at 2 years postoperatively. They reported preoperative severe flexion contracture and epidural anesthesia as the risk factors for the development of the nerve palsy in this patient.
Treatment and Results
The treatment of chronic peroneal nerve palsy usually consists of an ankle-foot orthosis for a footdrop and passive ankle ROM to prevent an equinus deformity. Complete recovery of a peroneal palsy is rare. The recovery seen is usually partial, with sensory deficits that may be permanent; residual motor deficits are not usually of clinical significance. Occasional marked weakness, especially of the great toe extensor, may be seen. In Asp and Rand’s study6 of 26 postoperative peroneal palsies, palsies were complete in 18 and incomplete in 8. Of these patients, 23 had motor and sensory deficits and 3 had only motor deficits. At 5-year follow-up, recovery was complete for 13 palsies and partial for 12. Complete recovery was more likely in palsies that were initially incomplete.
Although most investigators support nonoperative treatment of this complication, others disagree. Krackow and colleagues129 treated five patients with operative exploration and decompression of the peroneal nerve for a postoperative palsy. The procedure was performed 5 to 45 months after the index TKA. All patients have shown improved nerve function, and 4 of 5 patients had full peroneal nerve recovery. All patients were able to discontinue their ankle-foot orthosis.
Mechanical Complications
Instability
Instability is a common cause of mechanical failure of TKA. Instability accounts for 10% to 22% of TKA failures requiring revision.* The direction of instability at the tibiofemoral articulation can occur in the coronal (varus-valgus) plane, sagittal (anteroposterior) plane, or as a combination of planes. Early instability may be a result of malalignment of the components, failure to restore the mechanical axis of the limb, imbalance of the flexion-extension space, intraoperative or postoperative rupture of the medial collateral ligament (MCL), or posterior PCL rupture with cruciate-retaining designs. Commonly, late instability is secondary to polyethylene wear. Asymmetrical polyethylene wear related to malalignment can result in relative lengthening of the collateral ligament on the involved side and subsequent coronal instability. In patients with cruciate-retaining knees, it is not uncommon for the PCL to elongate or attenuate. This can lead to progressive polyethylene wear and late sagittal plane instability. However, late sagittal plane instability is seen not only with cruciate-retaining designs, because significant wear or fracture of the tibial polyethylene post in posterior-stabilized knees may result in late sagittal plane instability.207 Instability after knee arthroplasty can be subdivided into three types—extension instability, flexion instability, and genu recurvatum.
Extension Instability
Symmetrical Instability
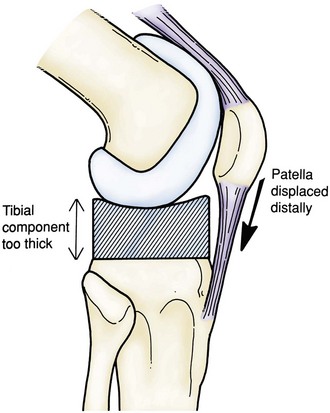
Symmetrical extension instability occurs when the extension space is not filled by the thickness of the components. This can be caused by overresection of the tibia or the distal femur.24 Although overresection of the tibia results in equally large flexion and extensions gaps, excessive resection from the distal femur results in asymmetrical flexion and extension gaps. If overresection of the tibia is recognized intraoperatively, it can be easily managed by increasing the size of the polyethylene insert. Similarly, if a postoperative TKA is noted to be equally loose in flexion and extension, one option is revision surgery with a polyethylene exchange to a larger insert. Overresection of the distal femur creates a much different scenario. With overresection of the distal femur, the joint line is elevated. This creates a larger extension gap compared with the flexion gap. By simply increasing the size of the tibial insert, the result will be a knee that is too tight in flexion. The elevated joint line will also affect patellar tracking and limit flexion, and may result in midflexion instability170 (Fig. 125-5). It is best in these cases to restore the joint line by adding distal femoral augmentation.
Asymmetrical Instability
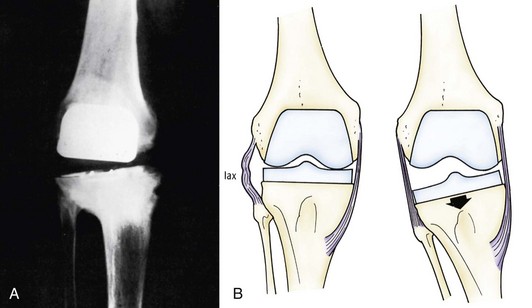
Asymmetrical extension instability is frequently encountered. Following the femoral and tibial preparation, the extension and flexion gaps must be assessed. An asymmetrical extension gap in the face of appropriate bony resection is most often caused by inadequate correction of the preoperative deformity.24,170 Preoperative varus deformity is the most common soft tissue deformity. The shortening or tightening of the medial structures, including the superficial MCL, must be recognized. Often, these tightened structures are not sufficiently released and the deformity is not completely corrected (Fig. 125-6). This will lead to progressing varus deformity with increased medial joint line stresses, accelerated polyethylene wear, and stretching out of the soft tissue structures on the lateral side. The asymmetrical extension gap must be recognized intraoperatively and an appropriate subperiosteal elevated of the superficial MCL performed, as described by Insall and associates.105 They thought that valgus instability from overrelease of the medial structures during correction of a fixed varus deformity was rare.
Knees with asymmetrical extension instability may also result from undercorrection of a preoperative valgus deformity.170 Excessively tight lateral structures, including the lateral collateral ligament (LCL) and iliotibial band (ITB), if left uncorrected, will lead to recurrence of the valgus deformity and increased lateral joint line forces. Currently, the most commonly used technique to correct preoperative valgus deformity without causing lateral instability has been described by Insall and associates105 as the “pie-crust” technique. This is recommended for patients with a preoperative valgus deformity of less than 20 degrees.3 Clarke and coworkers36 have published results of 24 TKAs with preoperative valgus deformity ranging from 9 to 30 degrees. The deformity was corrected using the “pie-crust” technique and the knee was reconstructed with a posterior-stabilized prosthesis. This series reported complete correction of the deformity and no instability in all cases. In patients with more than 20 degrees of valgus deformity, the risks of iatrogenic peroneal nerve injury must be considered.59 The surgeon may consider the use of a constrained condylar design in much older patients, whereas increased constraint in younger patients should be avoided unless absolutely necessary.
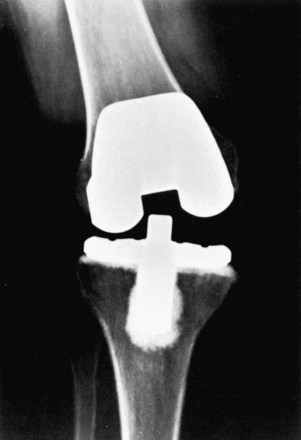
Extension instability can result secondary to iatrogenic injury of the collateral ligaments (Fig. 125-7). The excursion of large oscillating saw blades was studied and found to be greater than the width of the medial and lateral femoral condyles in the female knee.59 Meticulous attention must be paid to the position of retractors during femoral and tibial resection. Also, iatrogenic collateral ligament injury can be produced during aggressive testing of varus-valgus stability or with attempts to reduce the polyethylene insert in an excessively tight knee. With iatrogenic injury to the MCL, most authors advocate a direct repair with Krackow-pattern suturing. If primary repair is insufficient, augmentation can be performed using the hamstring tendons. Finally, a constrained condylar design can be used to provide additional stability while the repair heals. Several studies have demonstrated good outcomes without the use of increased constraint or tissue augmentation. Leopold and coworkers137 have demonstrated good to excellent results in 100% of 16 TKAs treated with direct repair of an iatrogenically injured MCL, reconstruction using a cruciate-retaining implant, and postoperative bracing for 6 weeks. More recently, Koo and Choi126 performed a retrospective review of 15 TKAs treated conservatively for intraoperative iatrogenic detachment of the MCL from the tibia. Specifically, no increased constraint, bracing, or ligament reconstruction was performed. No significant difference was seen between MCL-intact TKAs and the knees with iatrogenic MCL injury with a minimum of 2-year follow-up.
Flexion Instability
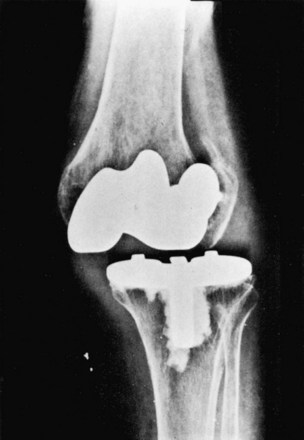
Flexion instability results from inadequate filling of the flexion gap or attenuation of the PCL following cruciate-retaining TKA. Flexion instability is now a well-recognized cause of TKA failure (Fig. 125-8). Early flexion instability is likely secondary to gap imbalance, with or without laxity of a collateral ligament170 (Fig. 125-9). Late flexion instability can occur in cruciate-retaining and posterior-stabilized TKAs, but the cause is different. In patients with cruciate-retaining knees, it is not uncommon for the PCL to elongate or attenuate. This can lead to progressive polyethylene wear and late flexion instability.167 Late flexion instability in posterior-stabilized knees may result from significant wear or fracture of the tibial polyethylene post.207 Symptoms can range from a vague sense of instability and recurrent effusions to frank dislocation. Flexion instability is best assessed with the knee in 90 degrees of flexion while the patient sits on the end of the examination table.
Frank dislocation following posterior-stabilized TKA is a rare complication204 (Fig. 125-10). Improved prosthetic design with increased jump distances have reduced this complication to well below 0.5%.170 Although the posterior-stabilized prosthesis can resist direct posterior translation, deep flexion combined with a varus or valgus stress can lead to posterior dislocation of the tibia. Patients who are at highest risk are those who have had the correction of a large valgus deformity and quickly regained their ROM during the postoperative period. The dislocated TKA can continue to function well following closed reduction under anesthesia; however, subsequent dislocation may occur. Recurrent dislocation requires revision to a larger polyethylene insert, if the extension gap permits, or component revision to a constrained condylar design.
More commonly, posterior stabilized knees may be symptomatic because of flexion instability but not demonstrate dislocation.204 Patients report a sense of instability, recurrent effusion, periarticular tenderness, and difficulty ambulating on stairs. The diagnosis is made on clinical examination with the knee flexed at 90 degrees. Schwab and colleagues204 have reviewed 1370 revision TKAs performed at the Mayo Clinic (Rochester, Minn). Ten revisions were performed for flexion instability. Eight (80%) had successful outcomes following revision of both components and appropriate balancing of the flexion and extension gaps. The excessively large flexion gap was frequently managed by upsizing the femoral component following posterior femoral augmentation.
Similarly, cruciate-retaining (CR) knees can be symptomatic because of flexion instability without dislocation. Patients again report a sense of instability, recurrent effusion, periarticular tenderness, pes anserine tenderness, and difficulty ambulating on stairs. Additionally, on physical examination, a posterior sag and positive posterior drawer may be readily apparent. Pagnano and associates167 have reported two types of causes for flexion instability following CR TKA. The first type creates an excessive flexion gap by surgical error. This can occur by undersizing the femoral component, which results in overresection of the posterior femoral condyles and a reduction in the femoral offset. Alternatively, the creation of excessive tibial slope can result in a knee that is well balanced in extension but remains loose in flexion. The second type involves late failure of the PCL and late instability. Other causes may include polyethylene wear, synovitis, iatrogenic PCL injury, traumatic PCL rupture, and an excessively tight flexion gap and PCL rupture following aggressive ROM exercises.
Treatment of CR knees with flexion instability most often involves revision arthroplasty to a posterior-stabilized (PS) design.170 At the time of revision, a larger femoral component is frequently used following posterior femoral augmentation. This improves posterior femoral offset and reduces the size of the flexion gap. Attention must also be paid to the tibial slope. If excessive posterior slope is present preoperatively, this must be addressed with revision of the tibial resection. Careful balancing of the flexion and extension gaps is then performed. Pagnano and associates167 have demonstrated successful outcomes following revision of a CR knee with flexion instability to a well-balanced PS design in 19 of 22 knees (86%).
Genu Recurvatum
Genu recurvatum, or hyperextension, is difficult to manage and therefore is best prevented during primary TKA. Because genu recurvatum is known to recur in patients with certain neuromuscular disorders, the cause of the hyperextension deformity must be elucidated thoroughly before surgery. In the absence of neuromuscular disease, however, hyperextension deformities tend not to recur after TKA. In the remainder of cases, recurvatum was likely present at the end of the index procedure as a result of excessively loose collateral ligaments and failure to fill the extension gap.148
Recurvatum before TKA is rare and is most often associated with neuromuscular diseases, including poliomyelitis.148 Patients with neuromuscular disease and significant quadriceps weakness rely on hyperextension of the knee to prevent limb collapse during the stance phase of gait. In the absence of neuromuscular disease, recurvatum may develop in patients with a fixed valgus deformity associated with a contracted ITB or in patients with rheumatoid arthritis. Patients at risk and those with preoperative hyperextension must be recognized so that appropriate considerations can be made.
Several options have been described to prevent the recurrence of recurvatum when performing a TKA in a patient with preoperative recurvatum. One option is to use a rotating hinged component with an extension stop. Giori and Lewallen76 reviewed the Mayo Clinic experience performing TKA in patients with poliomyelitis. They recommended the use of a rotating hinged prosthesis in patients with less than antigravity quadriceps strength. Jordan and coworkers111 more recently demonstrated 95% good to excellent results in 15 TKAs performed in patients with poliomyelitis using a PS or constrained condylar design. Unlike the results published by Giori and Lewallen,76 their results did not deteriorate with worsening quadriceps strength. Anterior-posterior stability was restored in all 15 patients. Only one patient required a hinged implant for reconstruction. The discrepancy in results remains unclear. Caution should remain when treating patients with less than antigravity quadriceps function.
A second alternative for patients with preoperative recurvatum is to underresect the distal femur. This will result in a relatively smaller extension gap. Then, by filling the flexion gap with the largest polyethylene insert possible, the knee will have a slight flexion contracture,170 which will prevent hyperextension. Finally, others have recommended repositioning the collateral ligaments more posteriorly to re-create the normal tightening action as full extension of the knee is achieved. The largest series available of patients with preoperative recurvatum without neuromuscular disorder has suggested that increased constraint, thicker components, and collateral ligament transfer are not necessary. Meding and colleagues149 published results of 57 TKAs performed on patients with at least 5 degrees of flexion contracture in the absence of neuromuscular disease. Using a CR design, they corrected the hyperextension deformity in 98% of knees. All but two knees (5%) reached full extension at the end of the procedure. With an average 4.5-year follow-up, only two knees had a hyperextension deformity. Both these knees demonstrated residual medial instability immediately following insertion of the prosthesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree