Classification systems
Cierny-Mader staging system for osteomyelitis
Stage 1: Medullary osteomyelitis
A Host: healthy
Stage 2: superficial osteomyelitis
B Host:
Bs: Systemic compromise
Bl: Local compromise
Bls: Local and systemic compromise
Stage 3: localized osteomyelitis
C Host: Treatment worse than the disease
Stage 4: diffuse osteomyelitis
• Factors affecting immune surveillance, metabolism, and local vascularity
• Systemic factors (Bs): malnutrition, renal or hepatic failure, diabetes mellitus, chronic hypoxia, immune disease, extremes of age, immunosuppression, or immune deficiency
• Local factors (Bl): chronic lymphedema, venous stasis, major vessel compromise, arteritis, extensive scarring, radiation fibrosis, small-vessel disease, neuropathy, tobacco abuse
Ger
Simple sinus
Chronic superficial ulcer
Multiple sinuses
Multiple skin lined sinuses
Weiland
Type I
Open with exposed bone without evidence of osseous infection but with soft-tissue infection
Type II
Circumferential, cortical, and endosteal infection. X-ray with diffuse inflammation and increased bone density, and spindle-shaped sclerotic thickening of cortex. Bone resorption and sequestrum with involucrum
Type III
Cortical and endosteal infection with segmental bone defect
• Chronic OM with wound with exposed bone, positive bone cultures, and drainage for 6 months. Soft tissue and location of bone involved
May
Type I
Intact tibia and fibula capable of withstanding functional loads
Rehabilitation time, 6–12 weeks
Type II
Intact tibia with bone graft needed only for structural support
Rehabilitation time, 3–6 months
Type III
Tibial defect <6 cm long with an intact fibula
Rehabilitation time, 6–12 months
Type IV
Tibial defect >6 cm and an intact fibula
Rehabilitation time, 12–18 months
Type V
Tibial defect >6 cm long without a usable intact fibula
Rehabilitation time, >18 months
• Focused on the status of the tibia after soft-tissue and skeletal debridement
Classifications of osteomyelitis are one small part of accurately defining the diagnosis and are lacking any merit in helping physicians determine etiology. It is the complexity of osteomyelitis that makes the initial diagnosis so challenging, and classifications do not completely nor clearly aid in defining the extent of the disease or guide precise treatments. The standard of care for osteomyelitis, by necessity, has therefore advanced beyond classification guided treatment in order to aid in appropriate early recognition and prevention of long-term consequences.
Risk Factors for Osteomyelitis
When considering all of the possible limitations in diagnostic techniques, physician understanding of associated risk factors for osteomyelitis plays a role in how a diagnosis of osteomyelitis may be made. These risk factors may include the patient’s age, gender, comorbidities, and exposure history. Exposures can be due to trauma, surgery, presence of tubes or external connectors, drugs, and more.
When considering age, acute osteomyelitis can be diagnosed at any age, but is the most commonly seen within the first to second decades of life. Conversely, chronic osteomyelitis frequency increases with age. Generally, patients under 18 years of age are more likely to have hematogenous osteomyelitis unless otherwise indicated [9]. On the contrary, bacteremia in adults rarely results in osteomyelitis, and secondary spread from a contiguous focus of infection, such as from surgical wounds or ulcerations, are more common. No studies have shown that age is itself a risk factor; however, patients who are over the age of 60 are more likely to suffer extreme complications from the disease, such as lower extremity amputations [10].
In regard to gender, no studies are conclusive of the most affected population. In one study, of 100 patients, there was found a 1:1 male to female ratio [10]. Another study, by Lavery et al., showed that of over 151 patients with lower extremity infections, the ratio was nearly equal, at 52 % male [11]. However, fungal osteomyelitis has been reported to have a statistically significant male dominance [12]. Gender should not be considered an independent risk factor and osteomyelitis can affect anyone. Overall, both age and gender cannot be used as a sole predictor of osteomyelitis; however, those with multiple comorbidities and increased age are at risk for a worse prognosis.
Specific comorbidities that weaken the host’s immune system increase the risk for a worse prognosis in the setting of osteomyelitis. A patient with diabetes has an increased risk for osteomyelitis, especially when there is poor glycemic control and presence of neuropathy. A retrospective cohort study of 8,905 patients found that 15 % of those with diabetes and a foot ulcer developed osteomyelitis at or after diagnosis [13]. Other medical conditions resulting in immunosuppression, organ transplants, or any condition that alters neutrophil defense, humoral immunity or cell-mediated immunity, can increase susceptibility to infection and increase the risk of undiagnosed osteomyelitis. Additionally, those with chronic immunosuppression are more likely to be diagnosed with fungal originated osteomyelitis [12].
Circulation disorders are another risk factor that may decrease host immune response and increase the potential of a deep infection. Secondarily, these conditions decrease laboratory markers of infection and increase the difficulty of diagnosis and treatment effectiveness. Peripheral vascular disease can be seen in patients with poorly controlled diabetes, smokers, sickle cell disease, and more. All of these conditions increase the risk for peripheral vascular disease, which in turn can greatly increase the risk of osteomyelitis and reduce the effectiveness of therapy [14, 15].
Obvious exposures, such as a previous history of osteomyelitis or open wounds, elevate the risk for osteomyelitis. Recent trauma or osseous surgery in any population is also a risk factor for osteomyelitis. A severe bone fracture or a deep puncture wound provides organisms a portal of entry into osseous structures or nearby soft tissues. In particular, surgical correction following open trauma involving osseous or articular surfaces increases patient risk in developing osteomyelitis [9].
Infected hardware is a devastating complication, especially with joint replacement procedures, carrying with it a poor prognosis for salvage of the implant. Specific factors related to poor prognosis in the setting of infected hardware include symptoms of inflammation, swelling, and pain to the site of the implant. The patients may also present with a history of a previous infection, upper respiratory tract infection, or bacteremia [16, 17]. Given the scope and severity of this topic, a later chapter is dedicated towards hardware and joint infections.
Patients who are on long-term external connections, external fixation, external tubing, or catheters and drains are also at greater risk for osteomyelitis. This includes patients associated with dialysis machines, urinary catheters, PICC lines or central lines, digital Kirchner wires, and external fixation devices for lower limb procedures (Fig. 2.1).
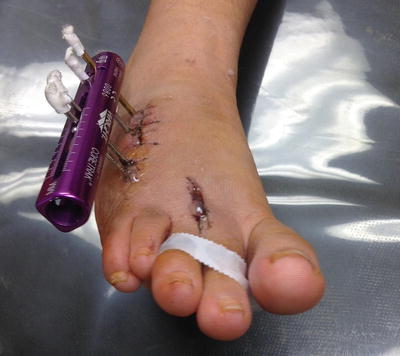

Fig. 2.1
Clinically infected monorail for the treatment of brachymetatarsalgia with portal of entry to the fourth metatarsal diaphysis
Patients who are exposed to injectable illicit drugs are also more likely to develop osteomyelitis. Illicit drug abusers typically use nonsterile techniques and have decreased immune systems due to prolonged abuse, making them not only susceptible to osteomyelitis, but to more resistant strains of bacteria. These potentially resistant but often polymicrobial infections can be found in up to 46 % of IV drug abuse cases [18].
Certain prescription medications may make a patient more susceptible to infection and simultaneously decrease inflammatory markers used in diagnosis of osteomyelitis. Immunosuppressive medications and medications that can mask symptoms of acute osteomyelitis include corticosteroids, tumor necrosis factor (TNF) inhibitors, antibiotics, chemotherapy agents, and more [12].
Different environmental settings are associated with a greater prevalence of osteomyelitis in certain patient subsets. Populations of hospitalized patients with a history of previous ulceration and infection have a higher prevalence of osteomyelitis than nondiabetic patients with open wounds seen in an outpatient ambulatory care clinic. In one study, 87.3 % of all hospitalized patients diagnosed with osteomyelitis had a previous history of trauma or skin infection [9]. But with all this considered, risk factors although helpful in gaining clinical suspicion, cannot be utilized as the sole diagnostic tool for lower extremity osteomyelitis.
Vital Signs
Initial evaluation of a patient with concerns for osteomyelitis should include assessment of vital signs. Vital signs should include temperature, blood pressure, heart rate, and respiratory rate. These markers are essential in evaluating the stability of a patient at risk for an acute infection. Infectious Disease Society of America (IDSA) guidelines specify that fever is defined as: (1) A single oral temperature >100 °F (>37.8 °C); or (2) repeated oral temperatures >99 °F (>37.2 °C) or rectal temperatures >99.5 °F (>37.5 °C); or (3) an increase in temperature of >2 °F (>1.1 °C) over the baseline temperature [19].
Fever or elevated temperature is a poor indicator of osteomyelitis due to its inconsistency and lack of specificity and sensitivity. Furthermore, the presence or absence of a fever should be interpreted depending on the specific population. Immunocompromised patients may have a normal temperature reading in the presence of severe infection as they may lack the systemic inflammatory response that a non-compromised patient would have. For similar reasons, patients with diabetes may have no fever present in the setting of acute and/or chronic osteomyelitis. Studies have found that up to 82 % of patients with diabetes have a normal oral temperature of <100.5 °F in the face of acute osteomyelitis. Sensitivity for fever and acute osteomyelitis was only 19 % and specificity could not be calculated as patients without osteomyelitis were not included [20].
Conversely, a pediatric patient may have a higher rise in temperature with a less serious medical condition. Yet, not all pediatric patients with osteomyelitis will have an elevated temperature. In one study, only 32 % of the pediatric patients presented with oral temperatures greater than 37.5 °C. All had confirmed cultured osteomyelitis of the lower extremity, but not all had elevated temperatures [21]. The type of organism may also influence body temperature. Ju et al. demonstrated that temperature appeared to be more elevated in those infected with MRSA vs MSSA, the difference being 38.8 °C and 37.2 °C, respectively [22]. When considering the pediatric and hematogenous group, the incidence of fever is also not an appropriate sole predictor for osteomyelitis.
Although fever is not a specific indicator of osteomyelitis, IDSA guidelines state that a temperature of >38 °C demonstrates a serious lower extremity infection with associate systemic inflammatory response and requires appropriate efficient treatment [19]. In the circumstance of an elevated temperature, the patient may have concomitant osteomyelitis, but this could be determined only in the appropriate clinical setting with adjuvant diagnostic criteria matching.
Clinical Signs and Symptoms
When considering clinical signs, the presence of inflammation, erythema, edema, and purulence does not independently determine the diagnosis of osteomyelitis. IDSA guidelines, however, do provide the clinician with a validated method for grading the severity of a general infection (Table 2.2) [19]. Despite this validated instrument, classic signs and symptoms of infection may be absent or masked by the coexistence of previously discussed comorbidities including vascular disease, diabetes, and neuropathy. Other conditions such as Charcot neuroarthropathy and gout may appear clinically similar to a patient with osteomyelitis.
Table 2.2
Infectious disease society of America (IDSA) guidelines on clinical appearance of infection [19]
IDSA Guidelines on clinical appearance of infection | Grade | IDSA infection severity |
|---|---|---|
No signs of symptoms of infection | 1 | Uninfected |
Infection involving the skin and the subcutaneous tissue | 2 | Mild |
At least two of the following items are present | ||
1. Local swelling or induration | ||
2. Erythema >0.5–2 cm around the ulcer | ||
3. Local tenderness or pain | ||
4. Local warmth | ||
5. Purulent discharge (thick, opaque to white, or sanguineous secretion) | ||
Other causes of an inflammatory response of the skin are excluded (e.g., trauma, gout, acute Charcot neuro-osteoarthropathy, fracture, thrombosis, venous stasis) | ||
Erythema >2 cm plus 1 of the items described above (swelling, tenderness, warmth, discharge) or infection involving structures deeper than the skin and subcutaneous tissues such as abscess, osteomyelitis, septic arthritis, fasciitis | 3 | Moderate |
No systematic inflammatory response signs, as described below | ||
Any foot infection with the following signs of a systemic inflammatory response syndrome | 4 | Severe |
Two or more of the following conditions: | ||
1. Temperature >38 or <36 °C | ||
2. Heart rate >90 beats/min | ||
3. Respiratory rate >20 breaths/min or Pa CO2 <32 mmHG | ||
White blood cell count >12,000 or <4,000/mm3 or 10 % immature band forms |
Hematogenous osteomyelitis occurs predominantly in prepubertal children. Patients usually present within several days to 1 week after the onset of symptoms, and therefore most often present in the acute phase. Patients often present with local signs of infection, including erythema, edema, tenderness to the involved bone, decreased range of motion, and warmth to the specific area. In addition, patients often have signs of systemic illness, including fever, chills, pain, irritability, and lethargy. Common locations affected by hematogenous osteomyelitis include the metaphysis of long bones, particularly the tibia and femur. In adults, hematogenous osteomyelitis is rare; however, the most common site is the vertebral bodies, followed by long bones, pelvis, and clavicle [2, 12].
Acute, subacute, and chronic forms of contiguous osteomyelitis more commonly occur in adults. Acute osteomyelitis is usually more representative of what is considered cardinal signs of infection: erythema, edema, tenderness to the bone involved (in the absences of neuropathy), decreased range of motion in the affected limb, drainage, and malodor. Chronic osteomyelitis may lack clinical signs and symptoms but is more likely in the presence of a chronic draining sinus tract, an open wound not responding to standard therapies, compound fractures, and wounds that have been present for a long period of time (Fig. 2.2). Contiguous osteomyelitis in patients without diabetes, usually do present with some signs and symptoms from a traumatic or surgical wound [23].
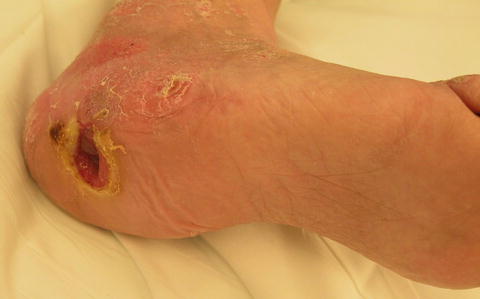

Fig. 2.2
Chronic nonhealing neuropathic diabetic heel wound with mild localized erythema and edema. Further evaluation revealed a sinus tract which probed to the calcaneus and radiographic changes consistent with osteomyelitis. Pathologic and microbiologic bone examination further confirmed the diagnosis of chronic osteomyelitis
In the diabetic foot, these infections almost always occur by contiguous spread, usually from a chronic ulcer. Osteomyelitis occurs in up to 15 % of patients with a diabetic foot ulcer and up to 20 % in those with infected foot ulcerations [13, 24, 25]. Osteomyelitis should be considered in patients with diabetes with a nonhealing foot wound, despite adequate perfusion and optimization of wound care and offloading [26]. In the setting of diabetes and peripheral neuropathy, infection occurs most often in the prominent bones of the feet, most frequently metatarsals. This is often due to biomechanical deformity, ill-fitting shoe gear, or an incident of trauma combined with peripheral neuropathy. Physical exam usually reveals some type of foot deformity alongside evidence of peripheral neuropathy (Fig. 2.3). The contiguous site of infection is typically a neuropathic ulcer, though paronychia from a toenail deformity, cellulitis or puncture wound are possible other sources.
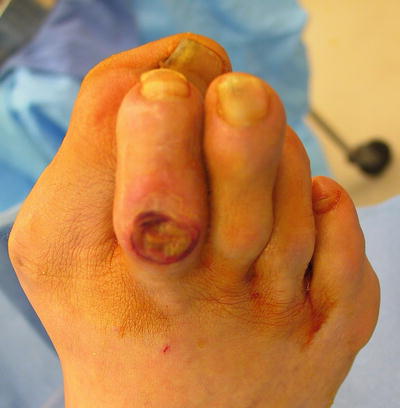

Fig. 2.3
The combination of forefoot pathology with peripheral neuropathy in a patient with diabetes is one of the most common pathways for the development of diabetic foot ulcers and eventual osteomyelitis
Wound Characteristics
Whenever there is an open wound, wound duration, size and depth should be assessed to aid in the diagnosis of osteomyelitis. Available evidence, however, does not justify using any one or combination of these wound characteristics as the sole criterion for diagnosing osteomyelitis.
Duration
A long-standing ulcer overlying a bony prominence, especially in the presence of adequate vascular supply, should raise the suspicion of underlying osteomyelitis if the wound is nonhealing and adequately offloaded. Calcaneal fractures, for example, are less likely to develop osteomyelitis if either traumatic and/or surgical wounds are able to heal within three months following initial injury [27].
Size
The association between the presence of osteomyelitis and the cross-sectional area of a wound has also been studied. The assumption has been that size of the ulcer has been considered predictive of osteomyelitis, however, the evidence to support this is limited and with conflicting evidence.
The diagnosis of underlying osteomyelitis on the basis of an ulcer area of ≥2 cm2 may have a sensitivity of 56 % and a specificity as high as 92 % [25, 26, 28]. However, another found that the wound area was ≥2 cm2 in 76.9 % of the patients with osteomyelitis and 69.2 % of the patients without osteomyelitis [29]. Another study found that increasing ulcer area had a protective effect with increasing ulcer size [30]. Measurement error is also a major concern with all studies assessing wound size. Most studies utilize measurements of the longest and widest diameters of a wound which do not reflect irregular shapes and depth [13].
These findings demonstrate that wound size is an unreliable guide to the presence of bone infection in a lower extremity ulcer. It can, however, be utilized as an adjuvant to the diagnosis for osteomyelitis.
Depth
It is thought that bone that is visible at the base of a wound is likely to be infected, especially when the clinical setting is appropriate. In a study where the prevalence of osteomyelitis was 68 %, the presence of exposed bone at the base of an ulcer had a sensitivity of only 32 % but a specificity of 100 % [25, 26]. Other studies have found that a wound depth greater than 3 mm was in 65–89.7 % of the patients with osteomyelitis but also in 80.8 % without osteomyelitis [29, 30]. Once again, it should be noted that accurate measurement of wound depth is difficult due to inconsistent measuring methods and irregular wound surfaces.
Depth via the Probe to Bone Test
In the absence of bone exposure, the probe-to-bone (PTB) test may be a useful diagnostic aid for osteomyelitis. While the PTB test is technically simple to execute, it is one of the most widely disputed clinical test for diagnosis of osteomyelitis. This is due to the challenges in properly interpreting existing clinical data on the PTB test.
To properly perform a PTB test, an examiner uses a sterile metallic blunt probe to gently probe the depth of a wound. If the probe touches bone, the finding is positive, whereas the inability to probe the base of a wound to periosteum, joint space, or bone is a negative result (Fig. 2.4).
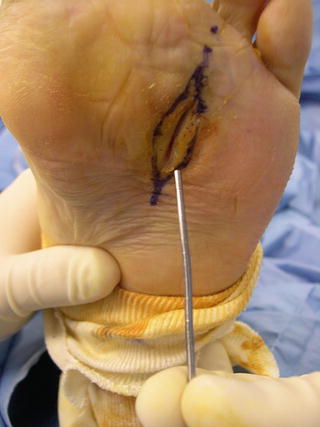

Fig. 2.4
To properly perform a probe-to-bone test, a metallic blunt probe is utilized to gently probe the wound base to ascertain whether or not a hard or gritty end point is felt indicating that a wound extends to bone, periosteum, or joint space
The first study to assess the PTB test was by Grayson and colleagues who examined a series of 76 hospitalized patients with limb-threatening infected diabetic foot ulcers on intravenous antibiotics. This was a nested retrospective cohort study from a previously performed randomized clinical trial. Diagnosis of osteomyelitis was based on histopathologic cultures and/or radiographic findings in combination with clinical criteria. The presumed prevalence of osteomyelitis in this population was 68 %. This study found that a positive PTB test had a sensitivity of 66 % and specificity of 87 %, giving a positive predictive value (PPV) of 89 % and a negative predictive value (NPV) of 56 % for the diagnosis of osteomyelitis [31]. The primary concern of this study is that because all wounds were from in-patient limb-threatening diabetic foot ulcers, the pre-test probability for osteomyelitis is much higher than wounds seen in the general population.
Another study with a high prevalence of osteomyelitis (72.5 %) evaluated the PTB test in 338 patients with 356 episodes of foot infection. Diagnosis of osteomyelitis was based on both bone histology and culture. Almost all patients required surgical debridement of osteomyelitis and therefore biopsies were taken at the time of surgery. The sensitivity, specificity, and positive and negative predictive values for a positive PTB test for the diagnosis of osteomyelitis were 95 %, 93 %, 97 %, and 83 %, respectively [32].
Conversely, two studies evaluated the PTB test in a patient population with a lower prevalence of osteomyelitis. Shone and colleagues examined the PTB test in 81 consecutive patients with 101 ft ulcers presenting to an out-patient multidisciplinary clinic. Inclusion criteria included both clinically infected and noninfected ulcers, and the prevalence of osteomyelitis was estimated to be 23.5 %. The diagnosis of osteomyelitis was determined from a blinded clinical examiner in combination with radiographs and MRI when indicated. Microbiologic bone specimens were collected in a small subset of patients. Researchers reported a sensitivity of 38 %, specificity of 91 %, negative predictive value of 85 %, and positive predictive value of only 53 % for positive probe-to-bone tests to diagnose osteomyelitis [33].
A prospective longitudinal cohort study evaluated the PTB test in 247 consecutive patients. Baseline prevalence of osteomyelitis was estimated to be 12 %, and all patients were seen in an out-patient-based specialty clinic. All infected ulcers had plain radiographs taken and additional imaging if deemed necessary. If osteomyelitis was suspected based on clinical exam and imaging modalities, histopathologic and microbiologic bone specimens were taken via aseptic technique to confirm the diagnosis of osteomyelitis. Patients with acute infections were not yet on antibiotics at the time of the PTB test examination. In this series, a positive PTB test had a sensitivity of 87 %, specificity of 91 %, negative predictive value of 96 %, and positive predictive value of only 57 %. The likelihood ratio for a positive test result was 9.40; the likelihood ratio for a negative test result was 0.14. This study emphasized that the probability of osteomyelitis among patients who had negative PTB test in this out-patient setting was very low [34].
To summarize, in order to properly interpret the PTB test, one must consider the population it is being performed within. The studies with the highest prevalence of osteomyelitis were referral centers (i.e., in-patient setting) with a population of ulcers that were infected and not responding to standard therapies. It was found that in a population with a high baseline prevalence for osteomyelitis, a positive PTB test had a high positive predictive value but a lower negative predictive value (Fig. 2.5) [31–33]. However, in studies examining the PTB test in a patient population with a lower pre-test probability of osteomyelitis (i.e., out-patient or clinic setting that included noninfected wounds), the PPV was much lower. Conversely, a negative PTB test had a high negative predictive value in these out-patient settings (Fig. 2.6) [33, 34]. In other words, the combined evidence of these studies suggest that the PTB test helps a clinician rule out osteomyelitis in an out-patient-based setting and rule-in osteomyelitis in populations with a high prevalence of osteomyelitis. These results confirm the importance of considering disease prevalence in assessing the PPV and NPV in diagnostic tests.
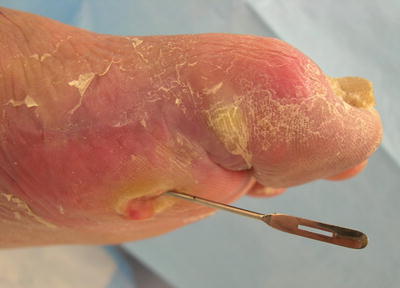

Fig. 2.5




Probe-to-bone test in an in-patient setting (high pre-test probability), making the positive predictive value of the PTB test very high
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








