By origin
Primary CHS (arising de novo)
Secondary CHS (arising from the cartilage cap of an osteochondroma, from sporadic enchondroma, or in skeletal enchondromatosis or osteochondromatosis)
By anatomic location
CHS of the axial skeleton (craniofacial region, ribs, spine, and pelvis)
CHS of the appendicular skeleton (long bones of extremities, hands, and feet)
By location within the bone
Central or intramedullary (usually arising in an enchondroma)
Central (intramedullary) conventional CHS
Peripheral CHS
Peripheral primary CHS (including periosteal/juxtacortical CHS)
Peripheral secondary CHS (usually arising from the cartilage cap of an osteochondroma)
Table 23.2
Characteristics of chondrosarcoma subtypes
Subtype | Frequency and gender predilection (M:F) | Peak age (years old) | Histologic grade (Lichtenstein and Jaffe classification) | 5-year survival rate (%)a |
|---|---|---|---|---|
Conventional (central and peripheral) | 85 % 1.5–2:1 | 40–60 | Low, intermediate, or high grade (grades 1–3) | 70 |
Periosteal or juxtacortical | <1 % Slight male predilection | 20–50 | Commonly low grade, rare intermediate, to high grade grades 1–3) | 93 |
Dedifferentiated | 10 % 1:1 | 50–60 | High grade (grade 3) | 0 |
Mesenchymal | 2–10 % 1:1 | 10–20 | High grade (grade 3) | 48 |
Clear cell | <2 % 2.5:1 | 20–30 | Low grade (grade 1) | 100 |
Secondary CHS may arise in a sporadic osteochondroma, in a genetic syndrome characterized by multiple osteochondromas (hereditary multiple exostosis), or in skeletal enchondromatosis (Ollier or Maffucci diseases).
The prognosis of CHS depends on the histologic grade, stage, and location of the tumor. According to the National Cancer Data Base Report, the relative 5-year survival rate of CHS is approximately 75 %, and patients who survive the first 10 years after diagnosis of conventional CHS will die of other causes rather than from CHS-related events. High-grade (grade 3) conventional CHS and dedifferentiated and mesenchymal CHSs account for those cases with less favorable prognosis. Extraskeletal myxoid CHS is not discussed in this chapter, because this tumor is a soft tissue tumor.
Primary Chondrosarcoma: Conventional Intramedullary Chondrosarcoma
Definition
Conventional CHS is a rare malignant tumor that shows cartilage differentiation. The tumor is usually found in the medullary cavity of a long bone but some arise on the surface of the bone.
Synonyms
Intramedullary CHS; Central CHS; Typical CHS; Chondrosarcoma, NOS
Etiology
The etiology of conventional primary CHS is unknown. However, several genetic abnormalities and molecular alterations have been recently found and are thought to be related to the pathogenesis and/or progression of CHS (see Genetics).
Clinical Features
Epidemiology
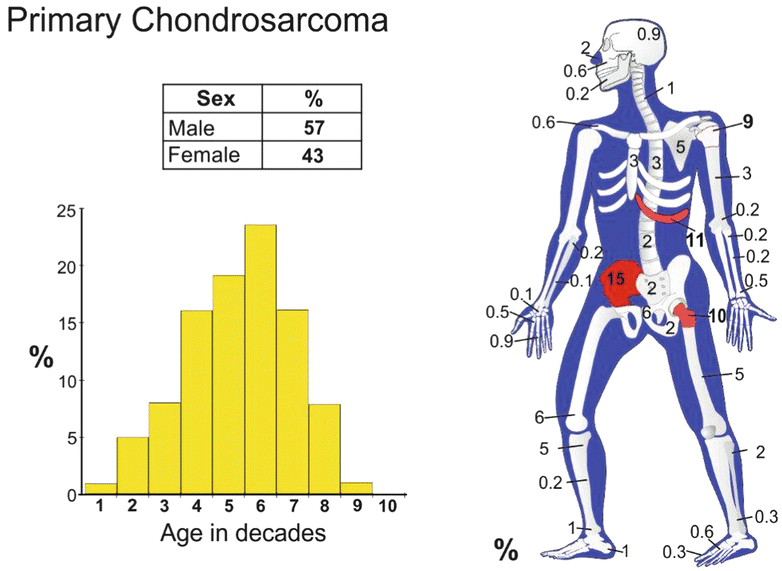
Conventional intramedullary CHS is the most common type of primary CHS (85 % of the cases). It is a tumor that predominantly affects adults from 30 to 70 years with a peak incidence at 40–60 years. The male to female ratio is 1.5–2 to 1. Conventional intramedullary CHS is very rare in children.
Sites of Involvement
Conventional CHS can present in any bone that develops by endochondral ossification. The frequent sites involved are the proximal femur, the iliac bone of the pelvis, the proximal humerus, the distal femur, and the ribs. Less frequent sites of involvement include the spine, the scapula, and the sternum. Craniofacial involvement as well as involvement of the neck, radius, ulna, clavicle, patella, and small tubular bones is rare. CHSs arising in specific anatomic locations are discussed below.
Clinical Symptoms and Signs
The clinical symptoms are usually nonspecific and may vary based on the size and aggressiveness (grade) of the tumor. The most common symptom of low-grade tumors is localized pain followed by local swelling (80 % of cases). Pain, however, may be related to osteoarthritis of a nearby joint.
Patients may also complain of a pulling sensation or restriction of movement of the adjacent joint. The symptoms usually have an insidious onset but may be progressive, being worse at night, and may last for months to years. Fast growing tumors may present with excruciating pain. Tumors from the pelvis usually produce urinary symptoms, such as frequency and/or obstruction, or may mimic a “muscle pull” in the groin. Pathologic fractures may be the initial presentation in less than a third of cases.
Image Diagnosis
Radiographic Findings
Plain films are still the best screening tool to determine if a lesion is cartilaginous in origin. Primary conventional CHS involving the long bones is more commonly seen in the proximal femur followed by the proximal humerus. In the femur, the intertrochanteric area, the metaphysis, and not uncommonly the diaphysis are the common sites involved. In the humerus, the epiphysis-metaphysis is commonly affected.
Typical radiologic findings include expansion and remodeling of the medullary cavity of the bone with endosteal scalloping and with or without periosteal reaction. The lesions usually show a lytic pattern typically with a background of punctate calcifications, referred to as “popcorn” or “ringlet” calcifications (Figs. 23.1, 23.2, and 23.3). Detection of these calcifications is diagnostic of cartilage tumors by imaging but is not sufficient to discern between a benign, borderline, or malignant chondromatous neoplasm. Enchondromas and intramedullary low-grade CHSs often share similar radiological features. A benign or borderline intramedullary tumor is favored when the lesion is small, lacks expansion of the medullary cavity, and shows absence of endosteal scalloping, cortical destruction, infiltrative pattern, or a lytic component (Figs. 23.1 and 23.2). In contrast, a suspicion for a high-grade intramedullary CHS should arise when a lesion is large, has produced expansion of the medullary cavity, and shows endosteal scalloping, especially if the lesion is painful (Fig. 23.4). Endosteal scalloping is a sign of aggressiveness in a central cartilaginous lesion but does not confirm the diagnosis of malignancy. Endosteal scalloping greater than two-thirds of the normal thickness of the long bone cortex is more predictable of CHS than enchondroma.
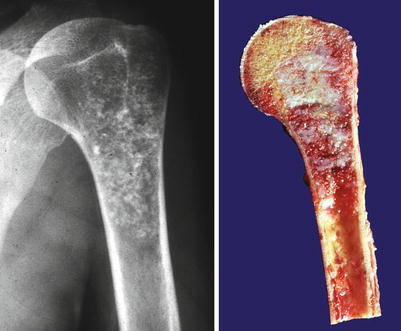
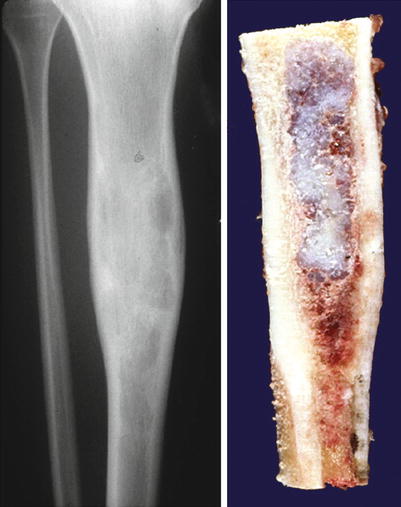
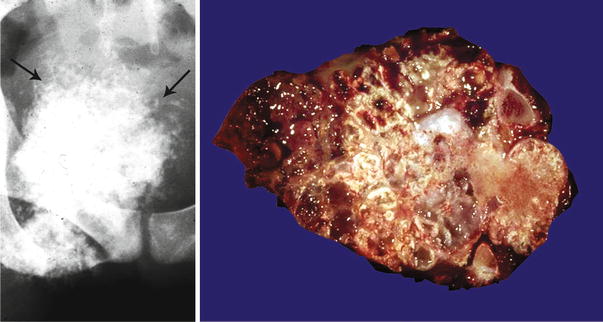
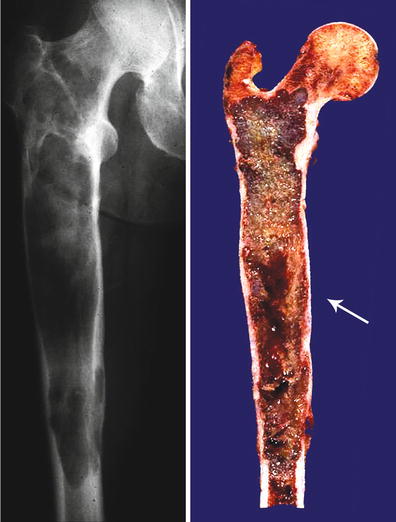

Fig. 23.1
Low-grade central CHS of the humerus. Left, the radiographic image shows the medullary cavity filled by a mass with punctate (popcorn or ringlet) calcifications. There is minimal endosteal scalloping and no cortical bone permeation. Right, same lesion after resection; the medullary cavity is not expanded but is partially occupied by a gray-white lobulated mass. This patient presented with pain in the shoulder that awakened her at night

Fig. 23.2
Low-grade central CHS of the proximal tibia. Left, the X-ray shows minimal expansion of the medullary cavity, endosteal scalloping with a periosteal reaction. Right, same lesion after resection; the medullary cavity is expanded and replaced by a gray-white lobulated mass

Fig. 23.3
CHS arising in the surface of the ischium. Left, the ischial spine is involved by a large cartilaginous lesion that shows prominent calcification including ringlet calcifications at the periphery (arrows). Right, same lesion after resection; the mass is composed of multiple lobules of cartilage calcified at the periphery, which correspond to the ringlet calcifications seen on the radiograph

Fig. 23.4
Central CHS of the femur. Extensive diaphyseal lesion extending into the intertrochanteric area. The medullary cavity is expanded, and there is endosteal scalloping but there is no breakthrough. Note that in the mid-diaphysis, there is an irregular area depicting myxoid change (arrow). These are signs of aggressiveness of the lesion, and the myxoid change strongly suggests a high-grade lesion
Features strongly suggestive of CHS include destruction of the bone cortex, extension into the adjacent soft tissues, and permeative changes (so-called moth-eaten pattern). Of these, only permeation is the most reliable sign of malignancy (Figs. 23.5 and 23.6).
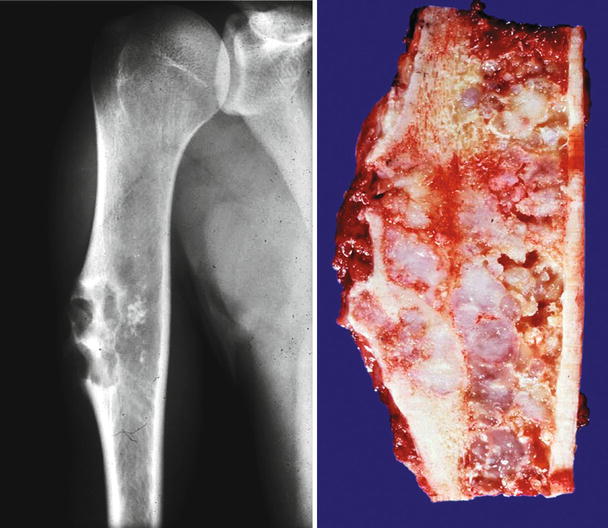
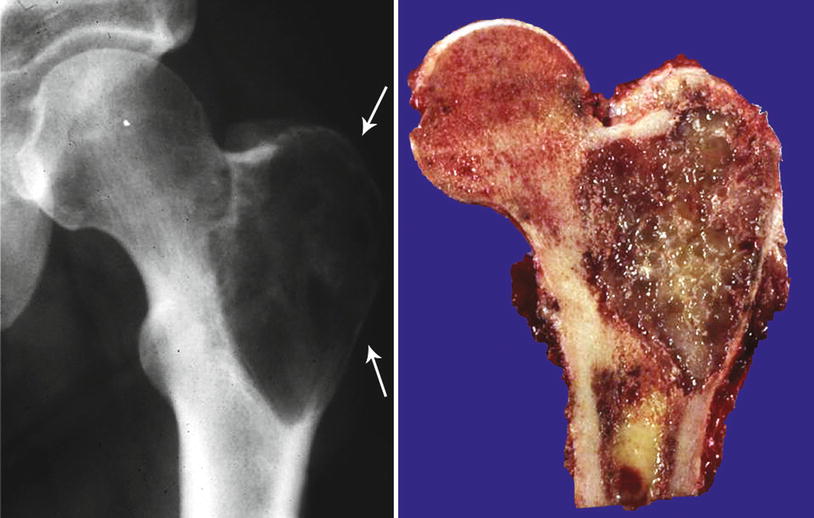

Fig. 23.5
Low-grade CHS with breakthrough of the cortex. Left, this tumor shows an aggressive behavior with involvement of the cortex. The bone shows a prominent periosteal reaction. Stippled calcifications are seen within the mass, indicative of a cartilaginous lesion. Right, same lesion after resection; the cortical bone is thickened with presence of cartilaginous lobules within the cortex, cortical disruption, and extension into the periosteum. Despite the involvement of the cortex, histologically, the lesion was low grade

Fig. 23.6
CHS with myxoid changes. This tumor is radiolucent and without calcifications. There is expansion of the trochanter and with bone cortical permeation (arrows). Right, the mass has marked myxoid changes. Minimal soft tissue periosteal extension is present. Bone permeation and myxoid change strongly suggest a high-grade cartilaginous neoplasm
Computer Tomography (CT) Features
CT and magnetic resonance imaging (MRI) scans are better modalities of imaging to evaluate the extent of the disease within or outside the bone. CT scan can evaluate the presence of cortical involvement, degree of endosteal scalloping, presence of soft tissue extension, and presence of lobulation and of matrix mineralization and can depict low attenuation rate due to the high water content of the cartilaginous matrix. In contrast, high-grade lesions may show increased attenuation due to the increased cellularity and less amount of water in the matrix.
Magnetic Resonance Imaging (MRI) Features
MRI provides an excellent way to evaluate the extent of marrow involvement and the presence of soft tissue invasion. Cross sections of the entire cortical circumference evaluate endosteal scalloping (two-thirds scalloping of the cortical thickness) as well as cortical remodeling and periosteal reaction. A recent study showed that formation of new periosteal bone reaction may occur in the absence of direct periosteal involvement by CHS of the femur, and thus, interpretation of tumor infiltration by MRI should be done cautiously. Likewise, CHS may extend into the soft tissues without evident findings on the MRI. Because of the high water content of the cartilage matrix in CHSs, marrow replacement appears as low to intermediate signal intensity on T1-weighted images and as high signal intensity on T2-weighted images (Fig. 23.7). Areas of matrix mineralization have low signal intensity in MRI sequences and are better visualized in plain radiographs or a CT scan. Low-grade CHSs show septal enhancement on gadolinium-enhanced MRI. High-grade lesions frequently present homogeneous or less frequently heterogeneous diffuse enhancement.
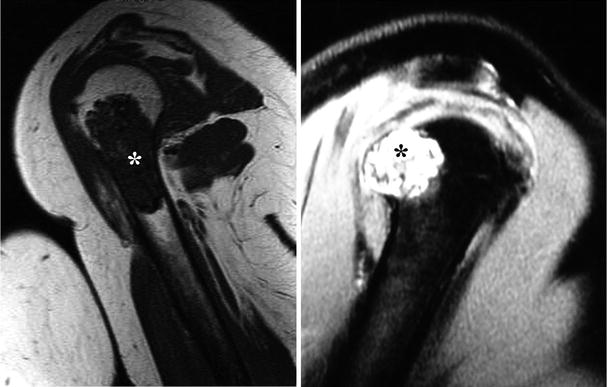

Fig. 23.7
Magnetic resonance imaging (MRI) is a better technique to evaluate the extent of marrow involvement and identify invasion into the soft tissues. On T1-weighted images (left), CHSs show low to intermediate signal due to the high water content of the cartilaginous matrix (asterisk). Compare the signal intensity of this lesion with the skeletal muscle around the humerus. On the other hand, CHSs have high signal intensity on T2-weighted images (right). This cartilaginous lesion is pushing through the cortical bone but does not invade into the soft tissues
Bone Scan
Approximately 80 % of intramedullary conventional CHSs show marked increase of radionuclide uptake (greater than the iliac spines) compared to enchondromas (20 % of cases). CHSs usually show heterogeneous radionuclide uptake.
Image Differential Diagnosis
As mentioned above, low- to intermediate-grade CHSs share similar radiological features with enchondromas. By location, low- grade CHSs are usually found in the axial skeleton and in flat bones compared to enchondromas which are more common in the appendicular skeleton. Inner cortical scalloping greater than two-thirds of the cortical thickness, periosteal reaction, cortical destruction, extension of an intramedullary mass into the soft tissues with peritumoral edema (better appreciated on water-sensitive MRI imaging sequences), and increased uptake of radionuclide at bone scintigraphy are all features that strongly suggest the diagnosis of CHS.
Pathology
Biopsy
As with any bone lesion, cartilaginous tumors can be approached with either closed or open biopsy procedures. Core needle biopsy (CNB) or fine needle aspiration (FNA) may yield sufficient diagnostic tissue for the identification of cell type, namely, in the case of cartilaginous tumors. CNB and FNA are done simultaneously and have the advantage of being easily done under local anesthesia utilizing radiologically guided imaging by an interventional radiologist. However, an open biopsy is equally effective but is costly and necessitates an operating room. Whether one technique or the other is elected for biopsy, the main disadvantage in biopsies of cartilaginous tumors is the sampling error that may lead into a wrong diagnosis. It should be stated that a tumor depicting typical calcifications (i.e., popcorn or ringlet type of calcifications) on radiologic examination is diagnostic of a cartilaginous tumor, so that there is no need to prove by histology that such lesion is of cartilaginous origin. For this reason, we do not advocate any type of biopsy for a lesion that can be recognized radiologically as being a cartilaginous tumor. However, when there are lytic areas within a radiologically typical cartilaginous tumor or permeation of the cortex strongly suggests a high-grade component, a CNB/FNA may have a role in the diagnosis. Such biopsy should target the suspicious areas for a high-grade component such as is the case of a mesenchymal CHS, dedifferentiated CHS, or a high-grade CHS.
Gross Features
Conventional intramedullary CHSs are tumors usually larger than 5 cm located in the medullary cavity of bones. Typically, the medullary cavity is expanded. Low- to intermediate-grade CHSs have a gray-white to bluish-gray cut surface and lobulated borders (Figs. 23.1, 23.2, 23.5, and 23.8). They usually have a firm consistency but may be soft, mucoid, or gelatinous or may even have a gritty cut surface with punctate calcifications (Fig. 23.3). Areas of endosteal scalloping and thickening of the periosteum are seen in long bones (Figs. 23.2 and 23.4). High-grade CHSs are gray-white and fleshy, with myxoid changes, hemorrhage, or necrosis (Fig. 23.6). The tumor usually extends from the medullary cavity into the adjacent soft tissues with infiltration and destruction of the cortical bone and periosteum (Figs. 23.9 and 23.10).
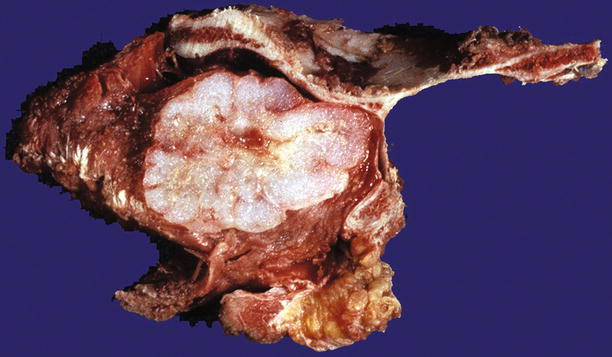
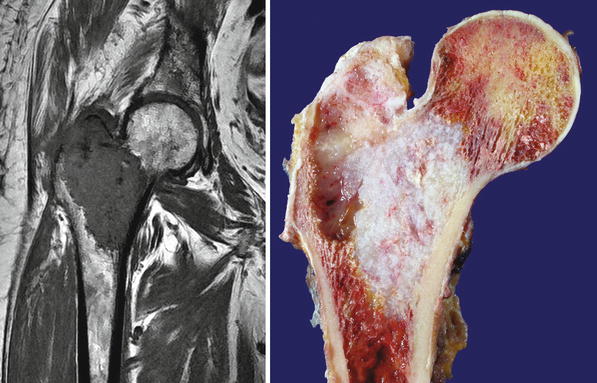
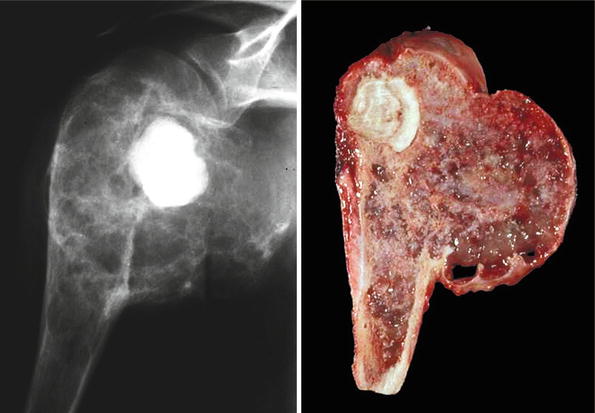

Fig. 23.8
Low-grade CHS of the scapula. Note the gray-white lobulated cut surface and stippled calcifications

Fig. 23.9
High-grade CHS of the femur. Left, the trochanter and intertrochanteric areas are occupied by a mass with similar signal as that of skeletal muscle. There is cortical bone permeation without a soft tissue mass. Right, the gross image shows a pearly white mass with myxoid change

Fig. 23.10
High-grade CHS of the humerus. Left, the mass occupies the head of the humerus and extends into the glenohumeral joint. Right, gross image. The mass has areas of cartilage as well as prominent myxoid changes
For the purpose of this discussion, the term “enchondroma” is not utilized for cartilaginous lesions of the long bones in adult persons (see below). However, we recognize that they exist.
Histology
The histologic features of CHSs are basically divided into two categories: benign/low-grade lesions and tumors that are cytologically malignant.
Borderline/Low-Grade Chondrosarcomas
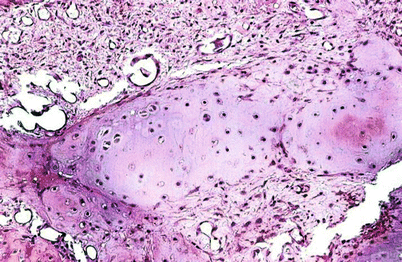
Benign/low-grade (borderline/enchondroma/low-grade CHS) cartilaginous lesions are difficult to assess histologically even when there is abundant tumor tissue. Histologically, these lesions are generally made up of well-differentiated hyaline cartilage without mitotic activity, pleomorphic nuclei, or significant cellularity (Fig. 23.11). Degenerative myxoid change may be present in these lesions and is characterized by myxoid change associated with pyknotic degenerating nuclei; in contrast, “neoplastic myxoid change” is defined as myxoid change associated with actively proliferating cartilaginous cells with definitive cellular atypia and partial or complete disappearance of the lacunar arrangement. Thus, benign/borderline or low-grade cartilaginous tumors are composed of lobules of hyaline cartilage with variable degree of cellularity and degenerative myxoid change and with or without calcifications. Chondrocytes usually show pyknotic nuclei with the size of a mature lymphocyte but larger nuclei may be present, especially in cellular areas. In the latter, the nuclei are enlarged and may contain a fine chromatin pattern and prominent nucleoli, but mitotic activity is not observed. Neoplastic myxoid change is not present. These cells are invariably located within well-defined lacunae (Figs. 23.11 and 23.12). Occasional double nuclei may be present but double nuclei have no pathologic significance since binucleate chondrocytes are commonly seen with the cartilaginous body of a callous from a fracture/repair or in the physis of growth in a child. In contrast, aggressive lesions radiologically demonstrating expansion of the medullary cavity and associated endosteal scalloping depicting benign/low-grade morphology as previously described are the crux of the radiologists and pathologists. The radiologist may read these lesions as “aggressive, cannot rule out CHS” or “consistent with low-grade CHS.” The pathologist may equally interpret the histology of this lesion as “borderline malignancy or low-grade CHS” (Fig. 23.5).
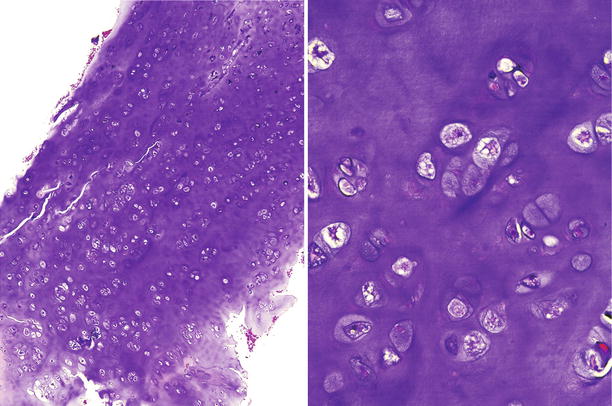
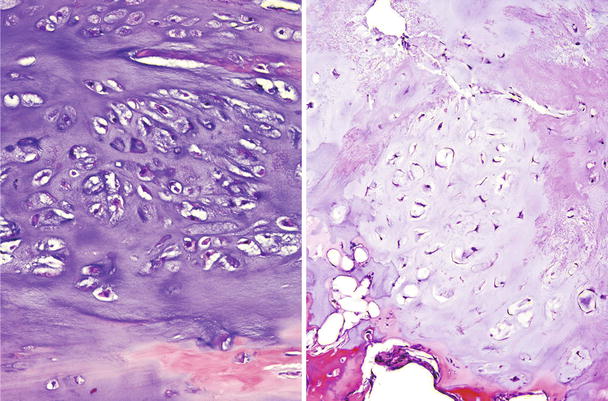

Fig. 23.11
Low-grade CHS. This lesion is characterized by a mildly cellular proliferation of mature cartilaginous tissue with cells in lacunar spaces. At higher magnification (right), the chondrocytes exhibit pyknotic nuclei, about the size of a mature lymphocyte without nuclear atypia, mitotic activity, or presence of stromal myxoid change

Fig. 23.12
Different morphologic spectrum of low-grade CHS. Left, this cartilaginous tumor shows mature cartilaginous matrix and more increased cellularity compared to Fig. 23.11. The cells have pyknotic nuclei with rare binucleate cells. Right, this other low-grade CHS shows mildly increased cellularity with chondrocytes retaining the lacunar formation and a more stellate morphology. No mitotic figures or myxoid change is seen in any case
Is a lesion an enchondroma? Or is it a borderline/low-grade CHS? This distinction is challenging, and many authors have attempted to define it. Thus, Mirra et al. felt that enchondromas are characterized by nodules of hyaline cartilage encased by marrow elements of lamellar bone. On the other hand, low-grade CHSs tend to infiltrate between bone spicules, replace marrow fat, and surround lamellar bone. Looking into Mirra’s illustrations of the low-grade CHSs, it becomes obvious that his tumors are depicting a neoplastic myxoid pattern and the cellularity is no longer made up of hyaline cartilage in lacunae but atypical chondroid cells (Fig. 23.13). Is it important to diagnose these lesions as benign or low-grade malignancy? The answer is probably not! These lesions should be surgically treated the same way.
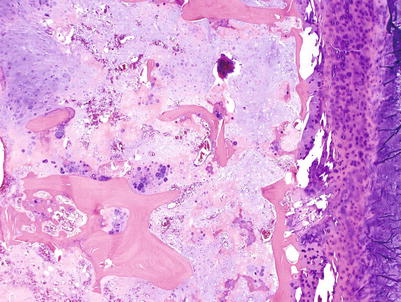

Fig. 23.13
CHS grade 2a. This cartilaginous lesion permeates between bone trabeculae and the lacunar arrangement, although still present, begins to fade and a myxoid stromal pattern begins to appear
High-Grade Cartilaginous Tumors
High-grade CHSs are not a diagnostic problem on histologic examination. They are characterized by a cellular cartilaginous proliferation of distinctively abnormal cells. Although there may be focal retention of the lacunar arrangement, this pattern is usually lost. The core of the tumor is made up of cytologically abnormal cells containing round, spindle, or pleomorphic nuclei and reduced amount of cytoplasm (Fig. 23.14). Mitotic activity is present but is not overwhelming. One has to look at many fields on a high magnification (×400) to find mitoses (Fig. 23.15). In Evans et al.’s grading system, the finding of two or more mitoses per ten high-power fields (HPFs) indicates a high-grade CHS. However, if a cartilaginous tumor shows many mitoses that are very easy to find, the tumor is most likely a chondroblastic osteosarcoma or a dedifferentiated CHS. Neoplastic myxoid change, namely, neoplastic cells separated by basophilic extracellular material with no lacunar formation, is usually present (Fig. 23.16). Extension of CHS through the Haversian canal-like channels into the soft tissues without obvious bone destruction may be observed, but such change is an extremely rare finding in the usual practice of pathology examination.
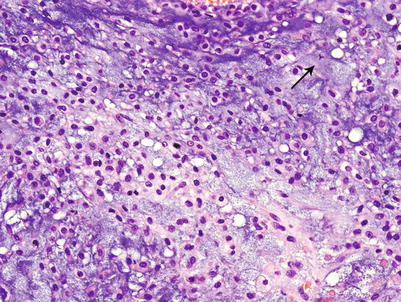
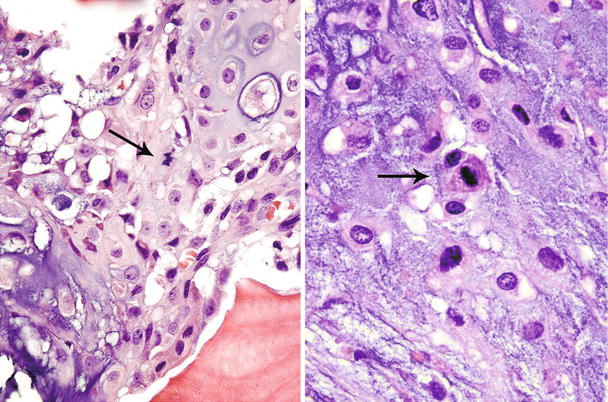

Fig. 23.14
High-grade CHS. Microscopic picture of the mass from Fig. 23.9. The tumor is composed of a hypercellular tumor with diffuse myxoid change, cells with nuclear enlargement, and occasional nucleolus. Note a tripolar mitosis in the upper right corner (arrow). The lacunar arrangement is completely lost

Fig. 23.15
High-grade CHS. Mitotic figures (arrows) are easily found in grade 3 CHSs

Fig. 23.16
CHS grade 2b. There is extension of a cartilaginous tumor between bone trabeculae. This cartilaginous tumor has lost the lacunar arrangement and shows diffuse myxoid change with cellular atypia. Myxoid change is not a feature of low-grade CHSs
So when there is definitive cellular atypia, increased cellularity, spindling, and the presence/absence of mitoses and myxoid change, the term CHS is justified.
Grading
There is a direct relationship between the histologic differentiation and the grade of CHSs. Grade 1 or low-grade CHSs are similar to benign cartilage lesions (enchondromas and osteochondromas) and are characterized by cells in lacunae without nuclear atypia and without mitotic activity.
Evans et al.’s grading system is a three-tier system (grade 1–3) where grade 1 lesions consist of a paucicellular proliferation of cartilaginous cells in lacunae (the size of a mature lymphocyte) with no nuclear atypia or mitotic activity (Figs. 23.11 and 23.12). Degenerative myxoid change characterized by relatively acellular myxoid with chondroid cells containing pyknotic nuclei may be present. Grade 2 lesions are cellular lesions that retain the lacunar pattern. The cells exhibit variability in the size of nuclei, which may have a fine nuclear chromatin pattern and a prominent nucleolus, and usually have abundant cytoplasm. However, there is no significant nuclear atypia or neoplastic myxoid change. Grade 3 lesions are cellular tumors depicting significant nuclear atypia and partial or complete loss of lacunar arrangement and must show ≥2 mitoses per 10 HPFs counted in the most cellular areas (Figs. 23.14 and 23.15). Two of the authors (A.G.A. and J.Y.R.) have refined this grading system by splitting the grade 2 into grade 2a and grade 2b (Fig. 23.17). Grade 2a lesions are cellular lesions with retention of the lacunar pattern, no nuclear atypia, and no mitotic activity. Grade 2b lesions consist of cartilaginous tumors that are cellular with partial or no retention of the lacunar pattern, none or one mitosis in ten HPFs, and characteristically exhibiting neoplastic myxoid change. Applying this grade system, grade 1 and grade 2a are tumors that may recur but do not metastasize. On the other hand, grade 2b and grade 3 lesions metastasize in about 60 % of the cases (Fig. 23.18, top). Grade 1 and grade 2a may recur, and the patient may die of uncontrolled local recurrence but such tumors do not metastasize. If one is to fuse grade 1 and 2a and grade 2b and grade 3, the survival curves are identical (Fig. 23.18, bottom). Therefore, well-differentiated low-grade lesions (grade 1 and 2a) may recur and grow but do not metastasize, while poorly differentiated high-grade lesions (grade 2b and 3) have a distinctive potential for distant metastasis.
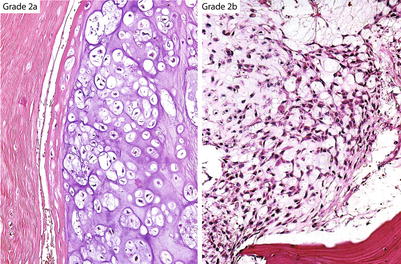
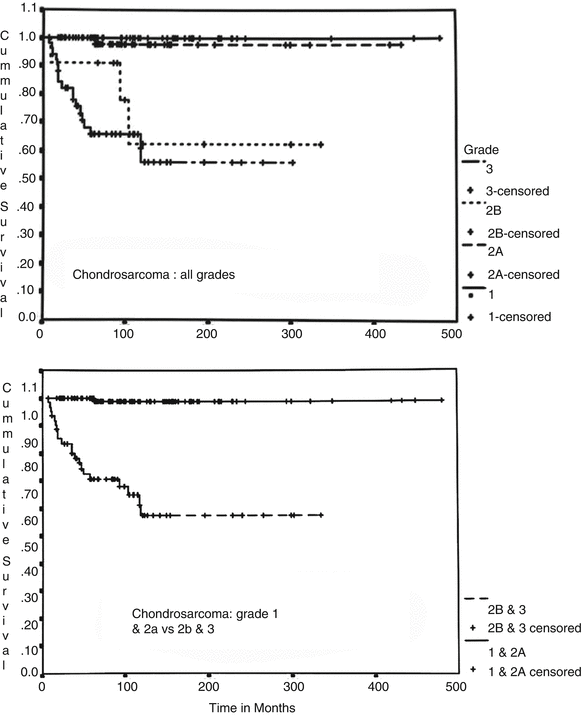

Fig. 23.17
Ayala’s subdivision of grade 2 CHSs. Grade 2a cartilaginous tumors maintain a lacunar arrangement and do not show nuclear atypia or mitotic activity. Grade 2b CHSs have increased cellularity, show partial or no retention of the lacunar pattern, may have one or no mitoses per ten HPFs, and contain myxoid matrix

Fig. 23.18
Cumulative survival charts in patients with CHSs. Grade 1 and 2a CHS and grade 2b and 3 CHS (top chart) are grouped into identical survival rates (see bottom chart). Grade 1 and 2a may recur but do not metastasize and have a good prognosis. In contrast, CHS grade 2b and 3 metastasize in about 60 % of the cases
In summary, high-grade CHSs have potential for distant metastasis and are characterized by the presence of at least two mitoses per ten HPFs, nuclear atypia, and neoplastic myxoid change. Mitoses, however, are difficult to find. Caution is given to the readers that if there are abundant mitoses, the tumor is most likely a chondroblastic osteosarcoma or a dedifferentiated CHS.
In our view, we prefer to interpret the benign/low-grade lesions (composed of purely hyaline “cartilage proliferation without neoplastic myxoid change or mitoses) as “cartilage tumor grade 1 or 2a” (depending on the cellularity) and comment that such lesion may recur but will not metastasize. In contrast, high-grade lesions (grade 2b and 3) will be called CHSs stating that they have definitive potential for distant metastasis.
Immunohistochemistry
In general, immunohistochemistry is not needed for the diagnosis of low-grade conventional CHSs. However, a useful marker of benign and malignant chondrocytes is the S100 protein. Several other immunohistochemical markers (Ki-67, MCM6, tenascin, A disintegrin, metalloproteinases, ezrin, telomerase) have been used to mark cartilage in an attempt to differentiate benign from low-grade cartilaginous lesions, but most of these studies need further investigation. Importantly, if osteoid material surrounded by atypical cells is recognized within the lesion, an osteosarcoma with chondroblastic differentiation and not a cartilaginous tumor should be suspected.
Ancillary Techniques
Genetics
CHSs show heterogeneous cytogenetic findings. Low-grade tumors show near diploidy, whereas high-grade tumors demonstrate more complex karyotypes. Cytogenetic abnormalities include several alterations including duplication or deletion of portions of or complete chromosomes as well as translocations. Single nucleotide polymorphism (SNP) arrays performed in CHSs have shown that these tumors first lose chromosomes and then duplicate their entire genome (autosomal loss of heterozygosity or LOH) as early events, and in later stages, the tumor cells acquire complex karyotypes after polydiploidization, additional gains, and losses or rearrangements of genetic material. Chromosome banding analysis and DNA flow cytometry has shown that deletions at 6q, 10p, 11p or 11q, 13q, and 22q loci are associated with impaired metastasis-free survival in CHSs. Overexpression of the protein p53 and mutations on chromosome 17 at the locus of the TP53 gene are present in almost all high-grade CHSs and suggest that mutation of p53 is a late event in the progression of this tumor. Amplification of 12q13 and loss of 9p21 are some of the more consistent genetic aberrations found in conventional CHS. The gene located in the 12q13 locus encodes the protein MDM2, a negative regulator of p53. The 9p21 locus harbors the genes of two cell cycle regulators, CDKN21/p16/INK4A and INK4Ap14ARF. Loss of p16/INK4A is restricted to high-grade CHS, suggesting a role in the progression of low- to high-grade CHS. Gene expression profiles of CHS (pre-chondrogenic and chondrogenic phenotype) have been established on the basis of chondrogenesis-relevant genes. This genetic profile permits distinction between grade 1 and grade 3 CHS and further stratifies grade 2 CHS in two groups, and a pre-chondrogenic or chondrogenic phenotype of a tumor could potentially be used as a good prognostic marker for clinical behavior.
In general, CHS follows the same parameters of tumor physiology including the self-sufficiency without the need of growth signals, resistance to growth inhibitory signals, anti-apoptotic mechanisms, uncontrolled proliferation, increased angiogenesis, invasion, and metastatic potential.
To date, several molecular markers and potential therapeutic targets have been identified for CHS, including the platelet-derived growth factor receptor (PDGFR), estrogen signaling, matrix metalloproteinase-1 (MMP-1), histone deacetylase, and vascular endothelial growth factor-A (VEGF-A). However, any of these markers have shown to correlate with the progression and behavior of CHS better than with the current histologic grading system. The reader is referred to more detailed reviews on the topic . The Hedgehog molecule, p53, cyclin-dependent kinase 4 (CDK4), hypoxia-inducible factor-1α (HIF-1α), several MMPs, the anti-apoptotic protein survivin, and SRC and AKT not only are important for the development of central CHS but also represent potential therapeutic targets. The Indian Hedgehog (IHH)/parathyroid hormone-like hormone (PTHLH, also known as parathyroid hormone-related protein or PTHRP) pathway normally regulates in a very delicate fashion the development of endochondral bone and maintains the growth of the endochondral plate at a constant thickness. Induction of IHH/PTHLH and reactivation of bcl-2, a downstream molecule of this pathway, are implicated in the pathogenesis and progression of conventional CHS, and bcl-2 may represent a reliable marker to distinguish between enchondroma and low-grade CHS (Fig. 23.19).
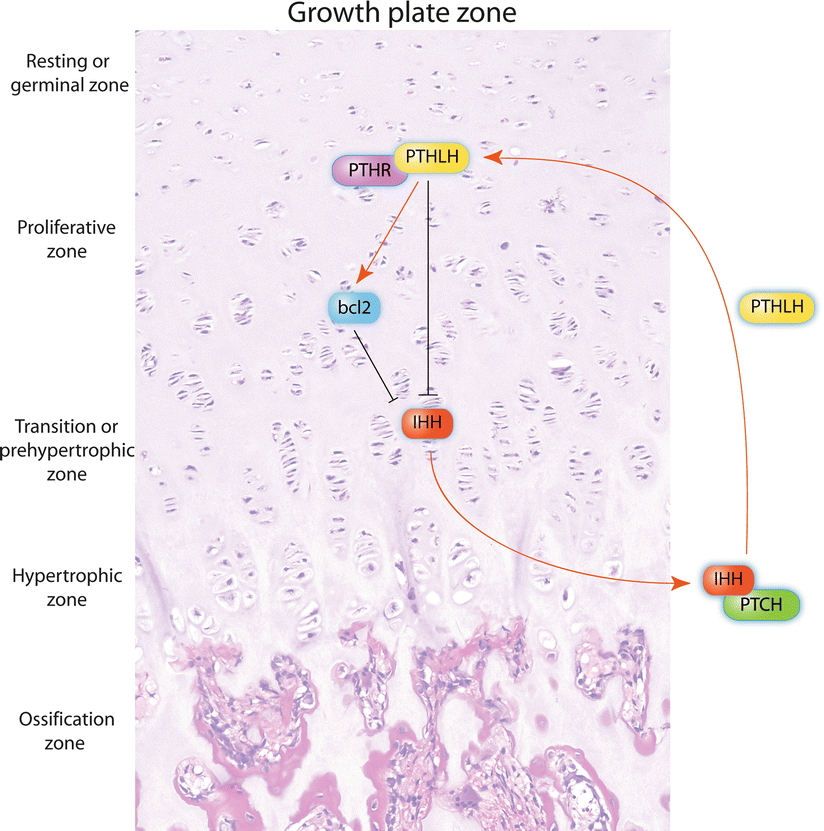

Fig. 23.19
The endochondral plate growth balance is regulated by the IHH/PHTLH pathway. Chondrocytes from the pre-hypertrophic zone produce Indian hedgehog (IHH) which binds its receptor Patched (PTCH) in hypertrophic chondrocytes to induce the production of parathyroid hormone-like hormone (PTHLH). The latter binds its receptor (PTHR) in chondrocytes from the resting zone and induces proliferation by different mechanisms (proliferative zone), including reactivation of bcl-2. As a regulatory feedback, PHTR-PTHLH downstream signals decrease the production of IHH, thus maintaining a constant thickness at the growth plate. In addition, increased bcl-2 is capable to decrease the production of IHH. These molecules travel through the different zones of the growth plate by diffusion (see also Fig. 23.27). Alterations in the IHH/PTHLH pathway and abnormal activation of bcl-2, among other mechanisms, are involved in the development of central CHS (Figure elaborated by Sergio Piña–Oviedo)
CHS differentiation may be reflected in the presence of particular types of extracellular components. Collagen types II and X and the proteoglycan aggrecan may represent markers of a mature neoplastic phenotype and good prognosis in CHS compared to collagen type I which indicates a transition to a more proliferative “dedifferentiated” phenotype. Hypermutability of the collagen II gene, COL2A1, which results in impaired collagen II synthesis, has been identified in about 40 % of CHSs by exome sequencing. The bone morphogenetic protein (BMP) and the transforming growth factor β (TGFβ) signaling pathways are active in central CHS cells, which could also represent important axis for the progression of CHS and as regulators of a “dedifferentiated” phenotype. Gene expression profiling in CHSs have shown that the protein JunB is higher in grade I CHSs compared to enchondromas and that CHS progression is associated with downregulation of matrix-related genes, increase in glycolysis-related genes, but decreased in oxidative-phosphorylation-related genes.
Overexpression of the enzyme cyclooxygenase-2 (COX-2, also known as prostaglandin-endoperoxide synthase) has also been proposed as a marker associated with high histologic grade and poor prognosis in CHS. However, the use of the COX-2 inhibitor, celecoxib, in CHS cell lines and a xenograft model of CHS failed to demonstrate complete remission of the tumor. Overall, however, none of these markers provides independent prognostic information.
In 2011, heterozygous somatic mutations of the isocitrate dehydrogenase 1 (IDH1) were identified in 56 % of benign and malignant cartilaginous neoplasms, except from peripheral CHSs and osteochondromas. About 40 % of the cases harbored the R132C mutation, which correlated with immunohistochemical detection of the IDH1-mutated protein in paraffin-embedded tissues. However, no correlation between IDH1 mutation and tumor grade was found. Importantly, the IDH1 mutation may represent a promising marker for the distinction between dedifferentiated CHS with osteosarcomatous differentiation and chondroblastic osteosarcoma. The enzyme IDH1 converts isocitrate to D-2-hydroxyglutarate instead of α-ketoglutarate with several consequences, including the activation of HIF-1α, an important molecule involved in cartilage proliferation, tumor angiogenesis, and cell proliferation in CHS.
Downregulation of several micro-RNAs has been detected in CHS compared to normal cartilage. Further studies are needed to understand the role of these novel molecules in the pathogenesis of CHSs.
Prognosis
Histologic grade is the single most important predictor of metastasis in CHS, with an inverse relationship between histologic grade and prognosis. Survival rates vary from study to study, and this may depend on the subjectivity of the interpretation of a cartilaginous neoplasm (Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones). The overall 5-year survival rate from the Surveillance, Epidemiology, and End Results (SEER) study was 70 %, close to the one reported by the Mayo Clinic on 77 % of 5-year survival rate for low-grade tumors. The overall survival rate for high-grade tumors is approximately 50 %. Nevertheless, a more recent study from Italy has shown that grade 1 CHSs have a 5-year survival of 92 %. Grade 2 and 3 CHSs are significantly associated with metastatic potential and have a 5-year survival rate of 77 %. Local recurrence occurs in 13–20 % of cases, and approximately 13 % of CHSs will recur as higher-grade lesions. Recurrence is higher in CHS of the axial skeleton. In a recent study of 115 patients with conventional intramedullary CHS, local recurrence and presence of distant metastasis was associated with a decline in overall survival regardless of the tumor grade. The presence of a pathologic fracture at diagnosis led to a decrease in overall survival in patients with CHS of the lower extremity but not in those tumors of the upper extremity. Also, the age of the patient (<40 years old), tumor volume (<100 cm3), and tumor location (appendicular vs. axial skeleton) were associated with better prognosis. Additional parameters such as tumor necrosis, mitotic rate, and inadequate surgical resection margins may be associated with poor prognosis.
CHSs of the appendicular skeleton have a better survival rate and lower recurrence in comparison with axial tumors. Our experience is similar. However, it should be stated that tumors of the extremities are easier to remove surgically than lesions of the axial skeleton.
Treatment
The aim of the therapy for a CHS is to resect the tumor regardless of the grade. However, the surgical approach has to be performed according to the clinico-radiologic presentation of the lesion. Surgery varies from curettage with bone or cement packing for non-aggressive lesions to en bloc resection with clean margins for aggressive lesions or malignant tumors. The key for success is to obtain clean surgical margins. The histologic examination is very important after the tumor has been resected because low-grade CHSs may recur if incompletely excised whereas high-grade (grade 3) tumors may develop distant metastasis. Thus, local recurrences are related to incomplete surgical excisions while metastases are related to the high grade of a lesion.
Chemotherapy and radiotherapy have no role in the primary management of CHS. However, radiotherapy may be utilized as a form of palliation.
Conventional Chondrosarcoma Located in Specific Anatomical Sites
As mentioned previously, CHS may be located essentially in any bone of the body (axial or appendicular skeleton) with involvement of the pelvis, ribs and sternum, the hands and feet, the vertebral spine, and the craniofacial region. Some of these tumors are discussed below.
Chondrosarcoma of the Hands and Feet
Definition
A conventional primary CHS that arises in the small bones of the hand or in the bones of the feet.
Synonyms
Primary CHS of the hands and feet; CHS of the phalanx; Phalangeal CHS
Etiology
The etiology of conventional primary CHS of the hand and feet is unknown. The tumor possibly shares similar genetic abnormalities as those seen in conventional intramedullary CHS. See Genetics for Conventional Intramedullary CHS.
Epidemiology
The development of malignant cartilaginous tumors in the hand and foot bones occurs in less than 5 % of all CHS cases, whereas enchondromas are extremely common. However, CHS is the most frequent malignant tumor in this location. In a study of 35 cases of phalangeal CHS, the median age at the time of diagnosis was 67 years (range 21–87 years) with a slight female predominance.
Site
This type of CHS develops far more commonly in the hand than in the foot. Within the hand, the fifth digit has the highest incidence of CHS and the fourth is the least involved. The proximal phalanges are the most common site affected. In the foot, the calcaneus is the bone most commonly involved.
Clinical Symptoms and Signs
Localized pain is the most frequent symptom at presentation. Fracture is unusual.
Image Diagnosis
Radiographic Features
CHSs of the hand and foot are radiolucent lesions (average size, 3 cm) with occasional punctuate calcifications that generally show destruction of the cortical bone with extension into the adjacent soft tissue (Fig. 23.20). Presence of a soft tissue mass is a more reliable way to discern whether a lesion is benign or malignant.
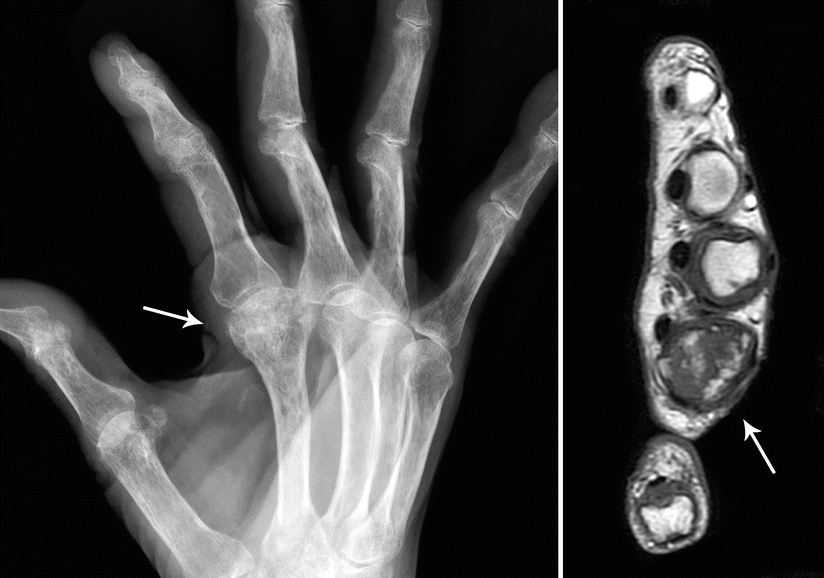

Fig. 23.20
CHS of the hand. Left, this radiograph of the right hand in oblique position shows a radiolucent lesion in the second metacarpal bone with punctate calcifications (arrow). Right, MRI at the level of the lesion shows and expanded metacarpal bone with heterogeneous intensities (arrow)
Image Differential Diagnosis
The main differential diagnosis is enchondroma. This benign lesion is confined to the medullary cavity of the hand and foot bones without cortical bone destruction.
Pathology
Gross Features
Similar to those described in conventional intramedullary CHS. Because of its location in small bones, the median size of these tumors is smaller (3 cm, range 1–8 cm).
Histologic Features
CHSs in these regions may be low- or high-grade tumors as seen in conventional intramedullary CHS.
Pathologic Differential Diagnosis
CHSs of the hands and feet are lesions that radiologically are clearly malignant showing definitive extension of the tumor from the medullary cavity into the adjacent soft tissue generally forming a large soft tissue mass. Histologically, they may be low grade or high grade (Fig. 23.21). Enchondromas are far more common in the bones of the hand than in the bones of the foot. They cause expansion of the medullary cavity and not uncommonly expand the entire length of a metacarpal bone. Enchondromas may present an associated fracture, but they do not show extension into the soft tissues. If a lesion does not show clear-cut evidence of high-grade malignancy, the best a pathologist can do is to call the lesion “cellular” or “atypical” refraining from making a diagnosis of CHS. While benign cartilaginous lesions may recur, recurrences are rare and take a long time to come back.
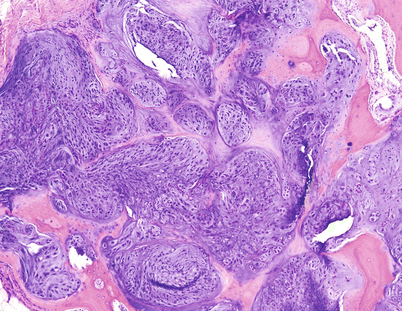

Fig. 23.21
Histologic image from the mass on Fig. 23.20. Low-grade CHS with hypercellularity and bone permeation
Ancillary Techniques
Genetics
The tumor possibly shares similar genetic abnormalities as those seen in conventional intramedullary CHS (see Genetics for Conventional Intramedullary CHS).
Prognosis
CHSs of the hands and feet are typically low-grade CHSs that may recur locally but do not metastasize. In a series of 35 cases of CHS of the phalanx, 10 of 15 tumors recurred after local therapy, whereas no tumor recurred after radical surgical resection. Conversely, CHSs of the calcaneus and talus bones are more likely to metastasize.
Treatment
If a complete curettage is diagnosed as a low-grade CHS, close follow-up is an option that the clinician has to make. If the curettage was incomplete, either a second curettage should be done depending on the radiologic presentation or a complete surgical resection with clean margins should be done with the latter option being curative in the majority of cases.
Chondrosarcoma of the Craniofacial Region
Definition
A conventional primary CHS that arises in the head and neck area, typically from the base of the skull.
Synonyms
CHS of the base of the skull; CHS of the head and neck; Sinonasal CHS
Etiology
The tumor originates from cartilaginous rests from the synchondroses of the basilar skull bones, which are formed by endochondral ossification during the fetal life. The tumor possibly shares similar genetic abnormalities as those seen in conventional intramedullary CHS (see Genetics in this section and Genetics for Conventional Intramedullary CHS).
Epidemiology
Craniofacial CHS represents 2 % of all conventional CHSs. In a study of 200 cases of CHS of the skull base, the age of presentation ranged from 10 to 79 years (median of 39 years) with a slight female predominance.
Site
As the name implies, craniofacial CHS is a tumor that arises in the base of the skull. The most frequently affected site is the temporo-occipital junction (66 %), followed by the clivus (28 %) and the sphenoid and ethmoid bones (6 %).
Clinical Symptoms and Signs
Patients develop neurologic and/or vascular symptoms associated with the region of the brain affected by the tumor growth (compression of the brainstem or of the cavernous sinuses). Swelling of the cheek and proptosis has been described in one case.
Image Diagnosis
Radiographic Features
Because of the complex anatomy of the craniofacial region, CT scan and/or MRI is the preferred method for the evaluation of skull base CHSs.
CT Features
CHS of the skull base shows cranial bone destruction usually with an associated soft tissue mass with areas of punctuate calcifications.
MRI Features
Bone destruction and a large soft tissue mass are better evaluated with this technique (Fig. 23.22). MRI is optimal for identifying areas of tumor extension but not to detect subtle matrix calcification. CHSs have lack of perfusion after administration of contrast compared to other tumors found in this location that enter the differential diagnosis, such as chordoma, meningioma, or metastasis.
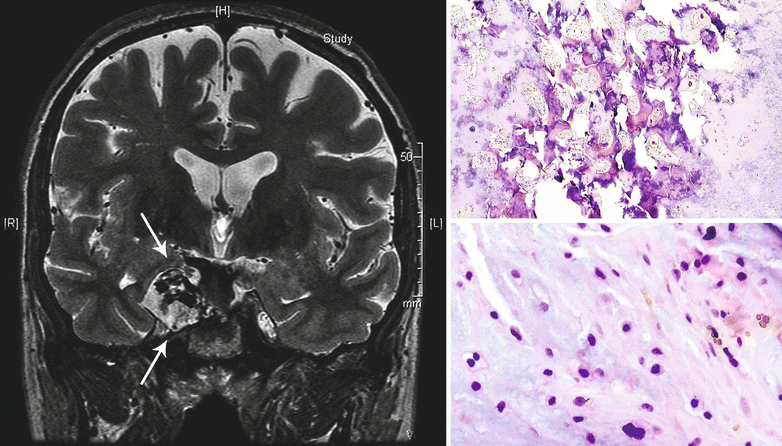

Fig. 23.22
CHS of the base of the skull. Left, the T2-weighted coronal MRI of the head demonstrates a mass with hyperintense signal involving the right wing of the sphenoid bone (arrows). Right top, the tumor is a low-grade CHS permeating into the bone. Right bottom, note the increased cellularity, focal myxoid component of the matrix, and the presence of binucleate chondrocytes
Image Differential Diagnosis
Chordoma arises in similar anatomic locations, particularly the clivus. Other tumors, such as osteosarcoma with extensive cartilaginous component, should be considered in the differential. A skull base meningioma or a metastasis should also be considered.
Pathology
Gross Features
Similar features to the description for conventional intramedullary CHS.
Histologic Features
In a study of 200 cases of CHS of the skull base, approximately 50 % of the tumors were classified as grade 1, 28.5 % as mixed grade 1 and 2, and 21 % as pure grade 2 CHS. No high-grade features have been reported in this region. Importantly, the majority of CHSs of the skull base produces a hyaline matrix with a myxoid component (Fig. 23.22).
Pathologic Differential Diagnosis
The main differential diagnosis of skull base CHS located in the clivus is chordoma. CHSs in this location commonly contain myxoid matrix and may resemble histologically the myxoid matrix seen in chordoma. In fact, some of these have been termed “chordoid CHSs.” Chordomas may also show focal production of cartilaginous matrix. Physaliferous cells are seen in chordoma but not in CHSs, but they may not be present in a small biopsy of chordoma. In this setting, immunohistochemistry for S100 protein, SOX9, pan-cytokeratin, epithelial membrane antigen (EMA), and brachyury may be necessary to establish the right diagnosis. CHSs are positive for S100 protein and SOX9 and negative for cytokeratin, EMA, and brachyury, whereas chordoma shows the inverse staining profile. Distinction between CHS and chordoma is crucial because CHSs have an excellent prognosis compared to the more aggressive course of chordomas. In addition, chordomas grow more rapidly and tend to occur a decade later than CHSs.
Ancillary Techniques
Genetics
The tumor possibly shares similar genetic abnormalities as those seen in conventional intramedullary CHS (see Genetics for Conventional Intramedullary CHS). The TGFβ and BMP pathways are activated in chondrocytes with resulting activation of ERK signaling, upregulation of MMPs, and destruction of the extracellular matrix, related to aggressive behavior. Similarly to conventional central CHS, IDH1 R132C mutations have been detected in 46 % of 13 intracranial CHS. This molecular test may be helpful to differentiate intracranial CHS from chordomas, which failed to demonstrate mutation of IDH1.
Prognosis
Craniofacial CHSs have an excellent prognosis. Both 5- and 10-year disease-specific survival are 99 %. Conversely, the 5- and 10-year disease-specific survival rates of chordoma are 51 % and 35 %, respectively. Tumors larger than 5 cm were associated with a lower 5-year survival rate in one study.
Treatment
The best treatment option is surgery and proton beam irradiation with survivals close to 100 %. From a review of the literature and analysis of 161 cases of sinonasal CHS, the use of radiotherapy for prevention of local recurrence after subtotal or total resection has not been effective, but the use of radiotherapy in addition to surgery has shown benefits in terms of survival.
Periosteal (Juxtacortical) Chondrosarcoma
Definition
This tumor is a low-grade primary conventional CHS that arises from the external surface of long bones, hence the name. It was originally described in 1955 by Louis Lichtenstein.
Synonyms
Periosteal CHS; juxtacortical CHS
Etiology
The etiology of periosteal CHS is unknown. The tumor possibly shares similar genetic abnormalities as those seen in conventional intramedullary CHS (see Genetics for this lesion and for conventional intramedullary CHS).
Epidemiology
Periosteal CHS is a rare type of CHS (<1 %) and represents <0.5 % of all bone tumors. The peak incidence is 20–50 years of age (earlier than conventional intramedullary CHS) with a slight male predominance.
Site
Periosteal CHS usually develops in the metaphyseal region of long bones, particularly the femur, the humerus, and less frequently in the tibia and fibula.
Clinical Symptoms and Signs
Periosteal CHS has no specific symptomatology. Most patients develop mild to moderate pain and a slow-growing palpable and painless mass in the affected region.
Image Diagnosis
Radiographic Features
Juxtacortical CHS tends to affect the metaphysis of the involved bone and is observed as a radiolucent mass adjacent to the cortex with well-defined cellular borders containing the typical “popcorn,” “ringlet,” or punctate calcifications (Fig. 23.23).
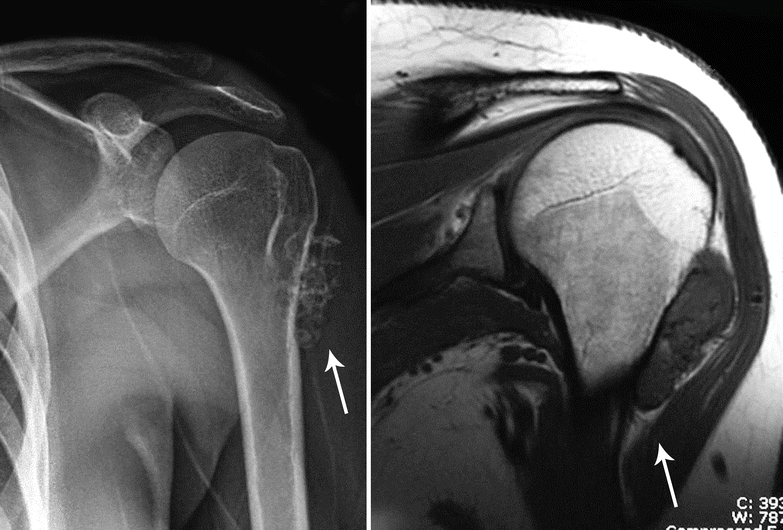

Fig. 23.23
Periosteal or juxtacortical CHS. Left, this oval-shaped tumor is located at the metaphysis of the humerus and has the typical features of CHS with ringlet calcifications (arrow). Right, on MRI, the lesion has a low to intermediate signal intensity on T1. Note the continuation of the tumor capsule with the periosteum of the humerus (arrow)
CT Features
This tumor is located adjacent to the cortex and has a round or oval shape. The cortex underlying the lesions may be eroded, thickened, or normal. Juxtacortical CHS has low attenuation due to the high water content of the cartilage matrix and may display calcific densities. As mentioned previously, matrix mineralization is better visualized on CT scan than on MRI. Peripheral enhancement is usually seen after contrast administration.
MRI Features
Juxtacortical CHS has well-delineated borders at the bone surface. Due to the high water content, the cartilage matrix shows low to intermediate signal intensity on T1-weighted images and bright signal on T1-weighted sequences (Fig. 23.23). Typical septal and peripheral enhancement is observed after administration of contrast material. MRI is the most sensitive method to identify intramedullary spread and/or soft tissue extension.
Image Differential Diagnosis
The differential diagnosis includes juxtacortical chondroma and periosteal osteosarcoma. The latter tends to involve the mid-diaphysis of a long bone, rather than the metaphysis. On X-rays, periosteal osteosarcoma is a small – rather than large – radiolucent mass with spicules of reactive bone perpendicular to the underlying cortex.
Pathology
Gross Features
Periosteal CHS is usually a large soft tissue round to oval mass with sharp borders with a mean size of 8 cm. The mass is covered by a fibrous pseudocapsule that is continuous with the underlying periosteum. On cut section, the tumor has a gritty cut surface and is white and lobulated with recognizable cartilaginous matrix and scattered calcifications (Fig. 23.24).
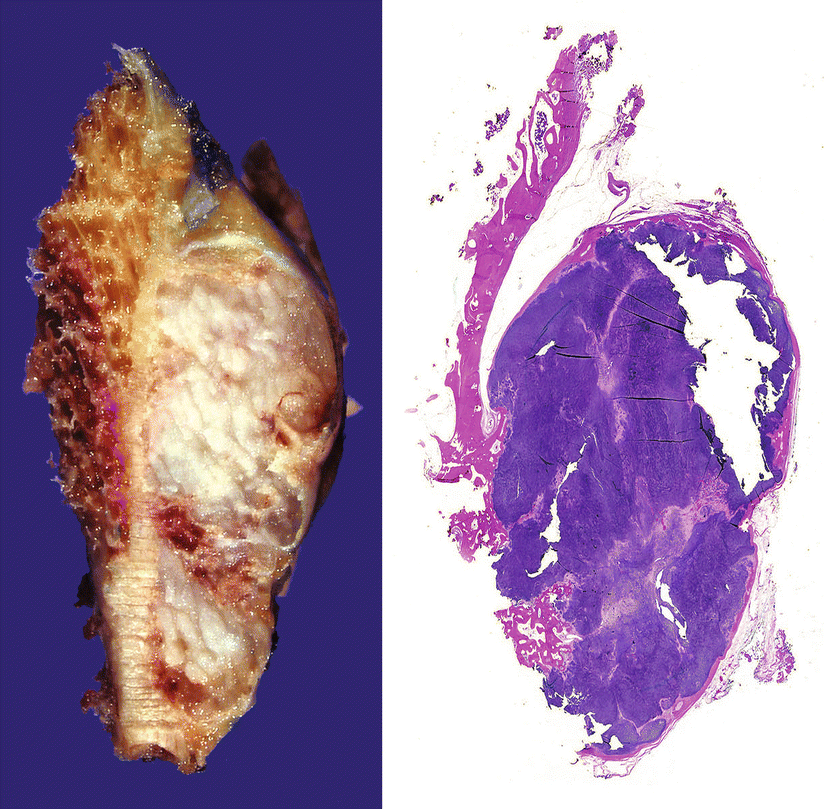

Fig. 23.24
Periosteal or juxtacortical CHS. Gross image (left) and full montage (right) of the lesion from Fig. 23.23. The tumor has well-defined borders, is surrounded by a capsule, and contains mature lobules of cartilage. Right, focal invasion into the bone is seen at the inferior aspect of the tumor
Histologic Features
Periosteal CHS is typically a low-grade tumor with similar features as seen in grade 1 or 2 CHS (Fig. 23.25). Despite the low-grade features, nodules of tumor can invade the surrounding soft tissues (Fig. 23.24, right). The amount of myxoid matrix varies from case to case. However, if osteoid matrix is present, the lesion should not be classified as a CHS as it may represent a periosteal osteosarcoma. Invasion into the medullary cavity of bone is not frequent and may occur by extension of the tumor through the Volkmann’s canal.