Chondral Injuries in The Knee
Onur Hapa
Alan F. Barber
INTRODUCTION AND OVERVIEW
Articular cartilage damage is common with sports-related injuries and often observed at arthroscopic surgery. Articular cartilage damage was found in 63% of more than 31,000 arthroscopies. The medial femoral condyle and the patellar surface were the most frequently injured sites (1). Although these articular cartilage lesions seem to be localized and limited in scope, what they represent for the future of the knee may not be innocuous.
The treatment of articular cartilage injury in the athlete presents several challenges. Not only does the average athlete wish to return to full activity as quickly as possible but the expectation that this will happen through the normal course of medical treatment also sometimes raises unrealistic expectations. In addition, more individuals are currently continuing their athletic activities longer, with the result that older patients are now included in the population sustaining athletic articular cartilage damage.
The knee articular cartilage has a complex structure and plays a vital role in normal athletic activity. It transmits loads uniformly across the joint and provides a smooth, low-friction, gliding surface. The lack of a vascular response and the relative absence of an undifferentiated cell population to respond to injury make damage to the articular cartilage a problem and limit its healing capacity. Localized full-thickness defects and contusions can cause significant symptoms and are especially problematic because of the potential for these lesions to progress. This is compounded by the natural environment of athletic participation during which the knee is repeatedly loaded, and the potential for violent contact with the ground or other participants exists.
Articular cartilage is a smooth, viscoelastic, hypocellular structure providing a low coefficient of friction. It has the ability to withstand significant recurring compressive loads. Articular cartilage has a large extracellular matrix composed principally of type II collagen (60% of the dry weight of cartilage) (2). The collagen fibers provide form and tensile strength, and water gives it substance by comprising 75% to 80% of the extracellular matrix. The cellular component (chondrocytes) synthesizes and degrades proteoglycans and is the metabolically active portion of this structure.
The articular cartilage injury can occur from shear forces associated with an anterior cruciate ligament (ACL) tear or blunt force trauma to the joint surface. This may result in the injury or death of articular chondrocytes. Although this can play a role in the development of articular cartilage degeneration after injury, such an injury may not be readily apparent at first. Areas of chondral injury and subchondral bone edema (bruising) are often seen on MRI with ACL tears.
The extent and implications of this injury may not be initially appreciated and may be one explanation for the late appearance of degenerative change after ACL reconstruction. If the articular cartilage is damaged, a defect may develop. Increased contact pressure is then placed on the edges of the articular cartilage defect and any exposed subchondral bone. This leads to overloading and degeneration of the defect with an expansion of the lesion. As this progresses, the exposed bone contacts the opposing articular cartilage, leading to bipolar injury and ultimately a bone-on-bone lesion.
The goal of the treatment of articular cartilage injury is to remove any tissue that is creating a problem and if needed replace it with durable tissue that not only fills the defect but also integrates well with the adjacent articular cartilage and does not deteriorate over time. One challenge of treating articular cartilage injury is its lack of a blood supply or an endogenous source of new cells, resulting in very limited spontaneous healing. The reparative process may result in fibrous tissue, degenerating hyaline tissue, fibrocartilage, or bone (3). The type of tissue created will determine the long-term clinical success. Factors affecting the repair quality include the patient’s age, lesion size, lesion depth, associated ligament instability, meniscus loss, angular malalignment, and the acuteness of the injury when treatment occurs.
CLINICAL EVALUATION
History
Chondral damage can be caused by various mechanisms of injury including a pivoting twisting fall, a direct impact on the knee, ACL tear, or a patellar dislocation. A traumatic hemarthrosis can be associated with chondral injury. Sometimes, no specific traumatic event is recalled and the patient only reports pain with weight bearing.
Pain is usually localized to one knee compartment. A persistent dull aching pain is often reported that worsens after activity and may be most noticeable when the patient attempts to fall asleep. Loaded activities such as running, stair climbing, rising from a chair, and squatting may aggravate these symptoms. Sitting for prolonged times such as in an automobile, a theater, or on an airplane may aggravate pain from a patellar lesion. In addition to pain, the patient may complain of swelling, crepitus, giving way, catching, and locking of the knee. This tends to be activity induced, but varies widely from patient to patient.
Physical Examination
Joint line tenderness, an effusion, and quadriceps atrophy are sometimes present, depending on the patient’s activity prior to the evaluation. It is important to evaluate limb alignment looking for varus or valgus change, hyperextension, or flexion contractures. For patellar or trochlear lesions, subpatellar crepitus, patellar grind, and pull-through sensitivity are often observed. Patellar tracking, the Q angle, and lateral retinaculum tightness should be evaluated. Associated lesions should be considered and meniscal signs and joint instability should be evaluated as well.
Imaging
A standard radiographic office evaluation should include the standing anterior-posterior view of both legs in full extension to look for angular changes and to compare joint space height. If this is not revealing, a 45° flexion posterior-anterior weight-bearing view to identify subtle joint space narrowing that the extension view does not demonstrate should be obtained. A nonweight-bearing lateral view obtained in 45° flexion in which the posterior femoral condyles overlap, an axial view of both patellae to help evaluate the patellar alignment, and an anterior-posterior knee flexion view to outline the femoral intercondylar notch should also be routinely obtained.
MRI can help outline the articular cartilage surface and demonstrate localized full-thickness lesions in a patient with otherwise normal standard radiographs. A layer of fluid or edema surrounding an articular cartilage lesion suggests that it may be detached. The two most widely used imaging techniques are the T1-weighted fat-suppressed three-dimensional (3D) spoiled gradient echo technique and the T2-weighted fast spin-echo technique. Software advances and newer MRI techniques with intravenous or intra-articular enhancement continue to improve the evaluation of articular cartilage.
Three-dimensional pulse sequence techniques are being used for preoperative assessments of articular cartilage defects to determine defect size and cartilage volume. The use of quantitative MRI to detect changes in the ultrastructure and biochemistry of articular cartilage is a developing technique for the evaluation of cartilage repair as well. These techniques assess either the proteoglycan content (sodium imaging, delayed gadolinium enhanced imaging, T1ρ mapping) or the collagen orientation (T2 mapping) within the cartilage repair tissue. In addition, higher field strengths (3.0-T magnets) with higher in-plane resolution are being used. Preoperative and postoperative cartilage assessment should be done with magnets having a 1.5-T strength or greater.
Classification
A consistent method of evaluating articular cartilage lesions is important to facilitate communication, arrive at a prognosis, and devise an appropriate treatment plan. This evaluation should consider the size and depth of the lesion as well as its location, any subchondral bone damage, and associated knee pathology such as an ACL or meniscal tear.
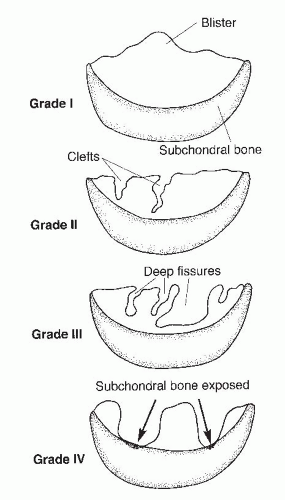
The Outerbridge classification system is commonly used for articular cartilage lesions (4). In this system, articular cartilage damage of grade 1 shows surface softening or blistering, grade 2 shows fibrillation or superficial fissures less than 1 cm in diameter, grade 3 shows deep fissuring extending into the subchondral bone without exposed bone, measuring more than 1 cm in diameter, and grade 4 shows exposed subchondral bone (Fig. 65.1). The modified International Cartilage Repair Society (ICRS) chondral injury classification system (5) was more recently developed (Fig. 65.2). This system is based on the depth and amount of the cartilage injury. ICRS grade 1 injuries are superficial with a soft indentation or superficial fissures and cracks. ICRS grade 2 lesions involve less than half of the cartilage depth, whereas ICRS grade 3 lesions involve half or more of the cartilage depth, but not into the subchondral bone. ICRS grade 4 lesions extend to include the subchondral bone.
Decision-Making Algorithms
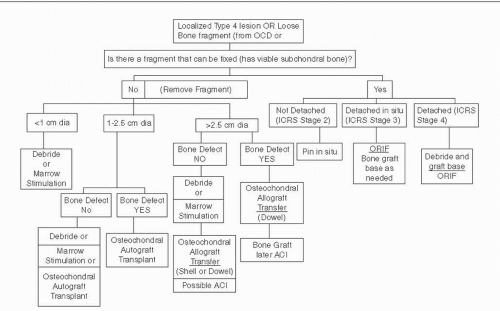
Articular cartilage treatment algorithms depend on the size and the depth of a lesion. The possibility for reattachment of a mobile fragment associated with the lesion exists only if the subchondral bone is viable. Some type IV lesions have associated bone loss. If the depth of loss is greater than 8 mm, marrow-stimulating techniques and autologous chondrocyte implantation (ACI) are not suitable choices and bone grafting to fill the defect should be performed either as part of an autograft or allograft procedure or as an independent initial step prior to ACI. Figure 65.3 outlines the treatment options based on these factors.
Osteochondritis Dissecans
Osteochondritis dissecans (OCD) is the term used for the separation of the articular cartilage with underlying
subchondral bony segment. It is divided into the juvenile (open physis) and the adult (closed physis) forms. Its etiology is unclear. It is more frequently seen in adolescent males with an increase in its prevalence and a decrease in the mean age of OCD patients noted in recent years. OCD is most commonly located in the posterolateral aspect of the medial femoral condyle. It usually manifests itself with vague, nonspecific symptoms. For diagnosis, comparative plain radiographs including the notch view are required. However, differentiating this condition from anatomical variations of normal ossification is difficult. An MRI helps distinguish between these two conditions and helps estimate the lesion size, status of the cartilage, and “stability of the OCD lesion” for deciding the treatment option. Treatment options consist of nonoperative (immobilization, isometric muscle strengthening, range-of-motion exercises, and nonweight bearing for 8 to 12 weeks) and operative treatment. The stability of the lesion is the most important consideration in selecting the appropriate treatment option. Size, weight-bearing surface, and the affected condyle are also important determinants of the prognosis. Operative treatment options include drilling of the OCD fragment in situ or operative fragment reduction followed by fixation using either Kirschner wires, compression screws, bone pegs, bioabsorbable screws, or fibrin glue. In the presence of a devitalized free fragment or a multifragmented piece that is not amenable to fixation, the techniques used for repair of focal chondral defects, such as microfracture, osteochondral autograft, autologous chondrocyte transfer, and fresh osteochondral grafting, can be used.
subchondral bony segment. It is divided into the juvenile (open physis) and the adult (closed physis) forms. Its etiology is unclear. It is more frequently seen in adolescent males with an increase in its prevalence and a decrease in the mean age of OCD patients noted in recent years. OCD is most commonly located in the posterolateral aspect of the medial femoral condyle. It usually manifests itself with vague, nonspecific symptoms. For diagnosis, comparative plain radiographs including the notch view are required. However, differentiating this condition from anatomical variations of normal ossification is difficult. An MRI helps distinguish between these two conditions and helps estimate the lesion size, status of the cartilage, and “stability of the OCD lesion” for deciding the treatment option. Treatment options consist of nonoperative (immobilization, isometric muscle strengthening, range-of-motion exercises, and nonweight bearing for 8 to 12 weeks) and operative treatment. The stability of the lesion is the most important consideration in selecting the appropriate treatment option. Size, weight-bearing surface, and the affected condyle are also important determinants of the prognosis. Operative treatment options include drilling of the OCD fragment in situ or operative fragment reduction followed by fixation using either Kirschner wires, compression screws, bone pegs, bioabsorbable screws, or fibrin glue. In the presence of a devitalized free fragment or a multifragmented piece that is not amenable to fixation, the techniques used for repair of focal chondral defects, such as microfracture, osteochondral autograft, autologous chondrocyte transfer, and fresh osteochondral grafting, can be used.
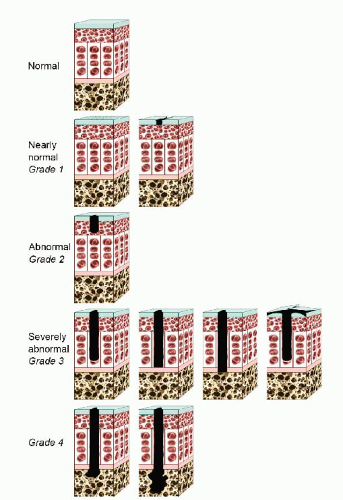 FIGURE 65.2. The ICRS chondral injury classification system. (Adapted from Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. j Bone joint Surg Am. 2003;85(suppl 2):58-69.) |
TREATMENT
Nonoperative
Arthroscopically, it is not uncommon to find Outerbridge type I or II chondral damage that does not require intervention. Smaller type III lesions that are asymptomatic can be observed and treated nonoperatively especially in athletes participating in low-impact activities. Small painful type III lesions may become asymptomatic once the acute synovitis resolves. Long-term studies suggest that isolated
chondral lesions less than 1 cm in diameter have an excellent or good prognosis without treatment and may be left alone (6). Intervention should be postponed as long as these areas do not cause symptoms.
chondral lesions less than 1 cm in diameter have an excellent or good prognosis without treatment and may be left alone (6). Intervention should be postponed as long as these areas do not cause symptoms.
Nonoperative care includes nonsteroidal anti-inflammatory drugs, physical therapy, activity modification, and possibly bracing. Bracing options include patellar-stabilizing braces for patellofemoral instability and load shifting shoe orthotics or knee braces for valgus or varus changes observed radiographically not responding to activity modification. Intra-articular injection of hyaluronic acid or steroid can be helpful.
Evaluating the particular position the athlete plays may provide opportunities for continued participation while “resting” the joint. Changes in an exercise program or technique may also prove helpful. Nonoperative treatment for an athlete is often based on the player’s position and the sport’s seasonal cycle. Operating on a player in the middle of his season can be a difficult choice and it is often better to wait until the season is over before surgery is performed. Although less than ideal, the future impact of such a choice on the knee’s prognosis and the potential consequences of the various treatment options should be thoroughly discussed with the athlete and the athlete’s family.
Operative Indications
The surgical indications for articular cartilage injury include significant, symptomatic type III and type IV lesions that have failed an appropriate nonoperative trial, the presence of loose bodies, and symptomatic OCD lesion not responding to nonoperative treatment. The goal of this surgery is to relieve symptoms including pain, swelling, catching, locking, and giving way, stabilize areas of irregular articular cartilage hopefully preventing further deterioration, to stabilize mobile or loose osteochondritis dessicans lesions, and to remove any unstable or loose fragments of articular cartilage and bone.
Unstable OCD fragment with viable subchondral bone or fresh traumatic fragments with viable bone can be primarily repaired (fixed to the bone bed). Articular cartilage lesions with loose osteochondral fragments (especially those >2 cm diameter) require immediate intervention to assess the potential to reattach a viable fragment. The chances of successful nonoperative treatment for these cases are low and the risks of progressive damage are high if allowed to go untreated.
TECHNIQUES
Debridement
Debridement of Outerbridge type III and IV lesions is an appropriate primary treatment for smaller lesions, especially in older, low-demand patients, and for those lesions associated with few symptoms (Fig. 65.4). The best results seem to be in younger patients with symptoms less than 1 year in duration, a specific history of trauma, no prior
surgery, little to no malalignment, and a low BMI (7). The debridement should only remove unstable chondral fragments causing mechanical symptoms and that are likely to become detached with additional activity or trauma.
surgery, little to no malalignment, and a low BMI (7). The debridement should only remove unstable chondral fragments causing mechanical symptoms and that are likely to become detached with additional activity or trauma.
 FIGURE 65.4. debridement of Outerbridge type III and IV lesions is appropriate for smaller lesions, low-demand patients, and incidentally observed lesions with few symptoms. |
Mechanical debridement does not stimulate articular cartilage repair, and there is a risk that the adjacent hyaline cartilage can be damaged (8). With additional trauma, these additional areas can progress to osteoarthritis. Mechanical debridement using a motorized shaver may use blades varying from an aggressive open-faced blade to one with small fenestrations (whisker blade) that is less likely to dig into normal articular cartilage. Damaged articular cartilage should be carefully probed to assess the extent of the softening and fragmentation before shaving. Only fragmented areas (Outerbridge type III) should be debrided. Any unstable edges of type IV lesions should also be debrided. On occasion, the shaver blade may not be able to completely debride a flap of articular cartilage and a basket punch is needed.
Once the extent and location of the lesion is appreciated, the shaver is inserted and brought into contact with the damaged articular cartilage. Little pressure on the shaver blade and a moderate amount of suction is required to perform this procedure. A back-and-forth sweeping motion is used to remove the prominent fragments. It is helpful to turn the cutting face of the shaver on its side so that the open face is at a 90° angle to the articular cartilage surface.
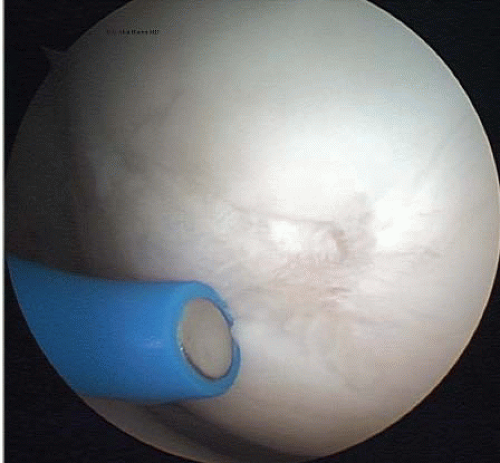 FIGURE 65.5. A monopolar thermal probe is used to treat Outerbridge type III damage on the medial femoral condyle. |
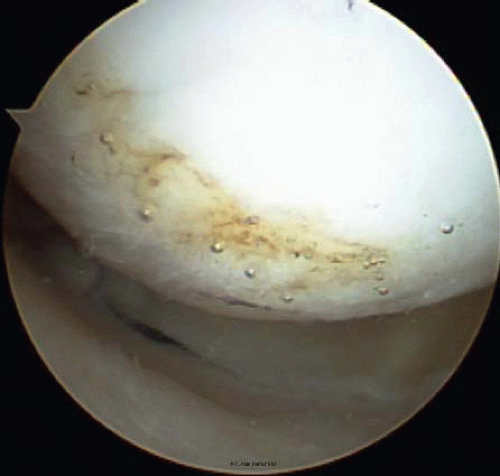 FIGURE 65.6. Thermal treatment of type III chondral lesions seals the cartilage, provides a smoother surface, and may prevent the extension of the lesion. |
The suction will lift the fragments into the rotating shaver blade that amputates them. Additional portals may be needed (especially for the patella) to address all the damaged areas without digging into the tissue excessively. Once a stable base is achieved, the debridement is terminated. The use of thermal treatment for type III damage has been advocated in the past but has fallen into disfavor (Fig. 65.5). Advocates of the thermal technique suggested that thermal treatment seals the cartilage, provides a smoother surface as seen with scanning electron microscopes, and may prevent the extension of the lesion (Fig. 65.6). If thermal treatment is selected, it should be applied to the area after the mechanical debridement is concluded. Concerns about heat damage to the adjacent macroscopically undamaged articular cartilage and subchondral bone have been raised. Treatment of the articular cartilage with heat causes immediate chondrocyte death. In addition, the type of heat application may be significant. Bipolar devices penetrate 78% to 92% deeper than monopolar systems and reach the subchondral bone when a
paintbrush pattern is utilized (9). Although concerns about the long-term safety and effectiveness of this technique have lead to a significant reduction in its use, there are some data which suggest it is more effective than shaver debridement (10).
paintbrush pattern is utilized (9). Although concerns about the long-term safety and effectiveness of this technique have lead to a significant reduction in its use, there are some data which suggest it is more effective than shaver debridement (10).
Rehabilitation
Progressive weight bearing is allowed starting immediately after surgery using crutches as needed. An aggressive exercise program is allowed, and depending on the extent of the articular cartilage damage, high-impact activities may begin as early as 3 to 4 weeks after surgery if the swelling has resolved and strength and pain permit. However, if full-thickness articular cartilage loss or extensive type III damage exists in the weight-bearing areas, high-impact activities such as running should be avoided.
Marrow Stimulation (Drilling, Microfracture, Abrasion)
Subchondral marrow-stimulation techniques have been utilized for many years. Initially this consisted of penetrating the subchondral bone with multiple drill holes (Pridie) (11) and later the technique of abrasion arthroplasty. Recently the use of variously angled picks was found to be easier than the more cumbersome drilling technique (microfracture). These techniques facilitate access to the vascular system and result in the development of a fibrocartilage scar that deteriorates over time. The initial reports of drilling were by Pridie11 who observed that treated areas became covered by a fibrous scar and resulted in clinical improvement. Later Johnson (12) advocated an arthroscopic variation of the technique called abrasion arthroplasty. This technique was principally indicated for extensive osteoarthritic knee surfaces. It required the abrasion of the entire surface removing 1 to 2 mm of bone in the involved area followed by a period of nonweight bearing of up to 8 weeks. Most of the subjects were older with degenerative arthritic changes and some reports of this technique showed poor clinical outcomes. This technique has few advocates today and was controversial even at its greatest popularity.
Penetrating the subchondral bone by either a drill or a pick is a common option for smaller localized areas (Fig. 65.7) (13). This technique provides a healing vascular response, leading to a fibrocartilage scar in the defect. Any chondral flaps are removed and the calcified cartilage layer is lightly debrided without damaging the underlying subchondral bone. Although there is no direct scientific proof about the exact origin of the healing tissue, the process by which this scar forms is thought by some to be through an influx of undifferentiated, mesenchymal cells from the subchondral marrow. It is recognized that this tissue response can be both unpredictable and variable. In addition, it is unclear whether this repair tissue responds well to compression and shear loads or whether it can withstand these stresses over time. The reported clinical results indicate an 80% “improved” status at an average of 7 years after treatment (13). However, no control group has been studied.
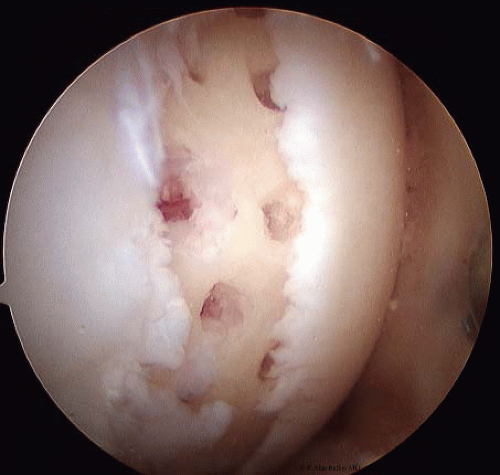 FIGURE 65.7. For smaller, more localized areas penetrating the subchondral bone by either a drill or a pick is a common option. |
Various angled picks are used for this technique (13) to perforate the subchondral bone. These picks are said to be superior to subchondral penetration by drilling because of less heat generation, which is suggested to be less destructive to the bone, by providing better access to the curved portions of the femoral condyle, a consistent depth of penetration, and the creation of holes that are perpendicular to the subchondral plate. Although the use of an angled pick certainly permits an easier access to the more posterior lesions, whether the depth and angle of penetration make any difference and whether, considering the cool aqueous arthroscopic environment, any significant heat difference exists between that created by the use of a pick and a small smooth drill is not established.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree