Chapter 18 Cervicogenic Sympathetic Syndromes
Cervical sympathetic ganglia, Horner’s syndrome, Barré-Liéou syndrome, Meniere’s disease, cervicogenic vertigo
After reading this chapter you should be able to answer the following questions:
| Question 1 | How does the position of the upper cervical ganglion make it vulnerable to an upper cervical subluxation? |
| Question 2 | How can a subluxation produce cervicogenic vertigo? |
| Question 3 | What is the role of manipulation and other forms of chiropractic adjustment in the treatment of cervicogenic sympathetic syndromes? |
The extremely flexible cervical spine, the body’s most complicated and mobile articular system, is located strategically between the head and body. It is designed for mobility, which significantly influences its stability. Subluxation syndromes affect both the mobility and stability of the cervical spine. The cervical spine is an anatomic complex of many crucial and sensitive tissues crowded into a small area. It is the “Times Square” of the human frame, with enormous volumes of traffic going in both directions.
Anatomy
This brief overview addresses anatomy relevant to injury of the cervical spine.
Sympathetic Nervous System
The middle cervical ganglion is the smallest and most variable in form and position. It is commonly located in close proximity to the inferior thyroid artery and the cricoid cartilage. In addition to supplying the gray rami communicantes to cervical nerves 5 and 6, it also gives rise to the middle cervical cardiac nerve.1
The superior cervical ganglion is the largest of the three, sometimes extending 3 to 4 cm; it lies opposite the transverse process of C2. This ganglion has many branches, including the internal carotid nerve, which distributes nerve fibers to intracranial vascular smooth muscles. Communications also exist between the superior ganglion and the inferior ganglion of the glossopharyngeal nerve, the inferior and superior ganglia of the vagus, and the hypoglossal nerve. Pharyngeal branches join the pharyngeal plexus as well as the external carotid and superior cervical cardiac plexus. In general, these fibers accompany the named blood vessels and perform vasoconstrictor, secretory, and pilomotor functions. The external carotid nerves supply all of the major and minor glands of the head and neck.2
The cervical sympathetic trunk lies deep to the deep layer of the cervical fascia and rests on the longus coli and longus capitis muscles. The nerve is posterior and medial to the carotid artery and the vagus nerve and usually ascends from its origin in the root of the neck superomedially.1
The anatomic intimacy between the sympathetic division of the autonomic nervous system and the somatic nervous system is most appropriate, because it is one of the main functions of the sympathetic nervous systems to continually tune visceral, metabolic, and circulatory activity to the rapidly changing requirements of the skeletal musculature.
Every motor activity organized through the somatic innervation originating in the spinal cord also involves the simultaneous coordinated activity of the sympathetic nervous system and the tissues and processes regulated by it. For the sympathetic nervous system to meet its supportive responsibilities to the musculoskeletal system, it must be continually apprised of the activities and requirements of that system. Hence, integration is possible only with simultaneous afferent input both to the motoneuron and to the sympathetic preganglionic neurons in the cord, from the higher centers through descending pathways, and from countless musculoskeletal reporting stations, through the dorsal roots.3 If there is a subluxation syndrome present at a particular level of the vertebral column, this affects the ability of the spinal cord to effectively integrate incoming and outgoing information. The rapid moment-to-moment adjustments in accordance with levels of exertion and posture—or anticipation, conscious or unconscious, of exertion—are orchestrated largely by the sympathetic nervous system.4
Sympathetic Effects in Healthy Tissue
If we accept that in several pathophysiologic states sympathetic efferents can produce pain and hyperalgesia, an obvious question is, to what extent can similar effects occur in normal healthy tissue?5 We know that many peripheral targets, in addition to blood vessels, receive sympathetic supply. For instance, there is good evidence for an innervation of muscle spindles, Pacinian corpuscles, and other specialized end organs in skin.6 We also might expect a modification of sensory inflow to occur secondary to changes in blood flow or piloerection.
It is therefore not surprising that physiologic experiments have identified some actions of sympathetic efferent in normal skin. Cold thermoreceptors can be excited by low-frequency stimulation of the sympathetic trunk.7 Similarly, some sensitive mechanoreceptors with unmyelinated axons are also transiently excited,8 an effect that is probably caused by small changes in the tension in the tissues secondary to vasomotor changes.9 A most important question is whether nociceptors are excited by sympathetic stimulation. Here all workers agree that for normal nociceptors with A, d, or C axons no direct excitation occurs.10–12 Together, these studies suggest that in normal tissue, sympathetic activity has only modest sensory effects.5 Would the presence of a subluxation complex change this modest sensory effect?
Sympathetically Mediated Pain
The most important conditions are those of causalgia and reflex sympathetic dystrophy. Causalgia is a condition that occasionally follows trauma to a major nerve. The term was coined by Mitchell in the middle of the nineteenth century to indicate the presence of persistent burning pain in such cases. The more general term reflex sympathetic dystrophy is used to categorize patients with some or all of the following symptoms: spontaneous burning pain, hyperalgesia (indicates both pain to normally innocuous events and increased sensitivity to noxious events), hyperpathia (delayed and exaggerated painful response to stimuli), disturbances of vasomotor and sudomotor control, and dystrophic changes in the peripheral tissues, such as abnormalities of hair and nail growth and osteoporosis. Although there is still much confusion in the use of these terms, reflex sympathetic dystrophy is generally used when the condition is not associated with obvious peripheral nerve trauma. Swelling is the most constant physical finding, which if not treated early is often followed by the rapid onset of stiffness.13 Precipitating events include minor tissue trauma and fractures, nontraumatic nerve lesions such as those seen in diabetes, and even lesions to the central nervous system. Could a subluxation syndrome be a factor in this condition?
There are two main reasons for believing that the sympathetic nervous system may be important in the genesis of pain in these conditions. First, sympathetic function is frequently abnormal. The skin of the affected region is often initially warm and red, later pale and cold. Anhidrosis or hyperhidrosis may be present. The dystrophic changes that occur are likely secondary to changes in blood flow to the area. A second and more compelling reason for implicating the sympathetic nervous system is that maneuvers that alter sympathetic activity frequently alter the patient’s pain. For instance, visual and auditory stimuli, emotional disturbance, or thermal stress all provoke sympathetic arousal and all can exacerbate the pain in these patients.5 A final important clinical finding is that pain often radiates extensively beyond the area of damaged tissue or the innervation territory of the damaged nerve. The spread usually ignores traditional boundaries such as nerve territories or dermatomes. In extreme cases, it can spread to encompass large areas of the body surface.
Central Changes
The ability of peripheral nerve lesions or peripheral tissue injury to alter spinal somatosensory processes is becoming well recognized. The ascending spinal pathways that transmit information rostrally begin to respond more vigorously to peripheral inputs and indeed can become responsive to totally new inputs that normally do not drive them. These cells also relay aberrant activity generated from the periphery. This process can develop quite rapidly with peripheral tissue injury, and it is possible that once established it may become largely independent of the precipitating event.14 The subluxation syndrome, if at the level of the injured peripheral nerve or tissue, affects the spinal somatosensory processing.
A second type of disturbance is seen in the response properties and patterns of reflex organization in the sympathetic nervous system. Within the dorsal horn, the representation of the body surface is very compressed, especially in the mediolateral plane. Expansion of receptive fields over quite substantial areas of the body surface is therefore possible and provides an explanation for the radiation of pain.5
Neck Proprioceptors
Proprioceptors provide information to the central nervous system (CNS). The receptors that send the information are specialized and located in extraspinal and spinal structures. Muscles, by their share of volume in the body, contain the most. The receptors are somewhat different and more numerous in the spinal musculature, especially in the cervical muscles. A review of the receptors, spinal muscles, and their spinal and CNS correlates has already been published.15 The essential points and new information related to the development of symptoms after trauma to the cervical area are covered in this section.
Muscle Receptors
Most muscle contains at least four types of receptors:
Muscle Spindles
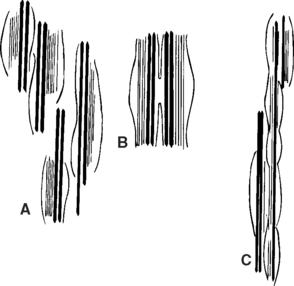
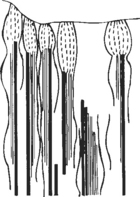
In the cervical musculature, the spindles are arranged in elaborate configurations such as paired, parallel, and tandem (Figure 18-1). Because of their volume per gram of tissue and their configurations, these spindles signal enormous volumes of information to the spinal cord and CNS when tiny changes occur in muscle. With a subluxation syndrome in the cervical spine, the information entering the spinal cord and CNS therefore is affected.
Golgi Tendon Organ
The Golgi tendon organ (GTO) is an encapsulated receptor that lies in series with extrafusal fibers at the musculotendinous junction (Figure 18-2). Most GTOs are located in the rostral half of the large muscles. In neck muscles, spindles and GTOs are often clustered together in complicated receptor arrays. Stretching of muscle, whether tonic or dynamic, causes the GTOs to inhibit the muscles.
Biomechanics
The essential biomechanics of the cervical spine, both upper and lower, are expertly reviewed by Moroney.17 Other pertinent information is presented here. There are five intervertebral discs (IVDs) from C2 through C7. The discs adhere above and below to hyaline articular cartilage, which covers the articular surfaces of the vertebral bodies. These five IVDs account for 25% of the total length of the cervical spine.18
Also reported were greater absolute and relative changes in the foraminal size at C6 and C7 foramens compared with the C5 foramen. This may be related to the relative flexibility of these levels. The study by Yoo et al.19 shows that the percent change of C5 foramen size was only 55% to 60% of C6 and C7 foramen size change, possibly reflecting a two-thirds decrease in flexibility. This relatively decreased motion may prevent greater excursion of the facets at the C4-5 interspace, accounting for the decrease in the alteration in the foramen size of C5.19
Age
In sagittal motion, there is an inverse relationship between age and range of motion; that is, as age increases, mobility decreases.18 Hayashi et al.20 compared three groups of healthy volunteers aged 20 to 40, 40 to 60, and 60 to 82 years. They found a 25% reduction in the maximum flexion and extension achieved when the group aged 60 to 82 was compared with that aged 20 to 40 years. Seventy-one percent of the decrease occurred at the C5-C7 motion segments. If there is a subluxation syndrome at these levels, it will further decrease the flexion-extension excursions.
Dvorak et al.21 showed that significantly decreased motion differences were found between age groups within gender and between gender groups in corresponding decades. Results of rotation out of maximum flexion suggest and support earlier conclusions that rotation of the C1-2 segment does not decrease with age, but rather increases slightly to perhaps compensate for the overall decreased motion in the lower segments. A subluxation syndrome at C1-2 therefore affects the overall motion of the cervical spine.
Intervertebral Discs
Pertinent information regarding the disc is thoroughly covered,22 and newer information is provided here. The major biochemical changes observed in the disc matrix with aging are similar to those described in other connective tissues. The most noticeable in the disc, however, is dehydration of nucleus pulposus (NP) and the loss of sulphated glycosaminoglycans (GAGs) accompanied by a large increase in noncollagenous proteins. The water content of the NP of the human disc decreases from 88% of dry weight at birth to 69% at 77 years. In the anulus fibrosis, water content declines from 78% at birth to 70% at 30 years, remaining relatively constant thereafter. The absolute amount of collagen also may increase with aging, but only slightly as a fraction of dry weight.
Numerous histopathologic studies of human discs at autopsy have confirmed that a high incidence of primary degenerative changes is present in individuals older than age 30, and this increases with age. However, retrospective investigations of the medical records of these individuals do not show a history of back complaints in all cases. This indicates that disc degeneration does not invariably lead to clinical symptoms of back pain. From these findings we may conclude that, with aging, biochemical changes take place within the matrix of the disc, which can reduce its ability to achieve efficient dissipation of the mechanical stresses imposed on the spine during everyday activities.23 A subluxation syndrome limits movement at a particular motion segment; this also affects the blood supply to the disc.
The nucleus pulposus of the human, like that of the dog, starts losing notochordal cells and depositing a hyaline-like matrix within a few years of birth. Because there is evidence in dogs that the rate at which this process takes place is genetically determined, it is not illogical to hypothesize that the disc cells of humans are similarly programmed. If this is so, then the response of discs within the human spine to restricted movement may, in part, be heritable. In animal experiments, surgical methods were used to restrict spinal movement; such procedures induced profound changes in disc metabolism within six months of application. In the case of the human spine, disc changes arising from inadequate nutrition may occur for several reasons. The subluxation syndrome may be initiated by insidious or traumatic circumstances, maintenance of bad posture such as sitting, driving, flying for long periods, lack of appropriate exercise, and smoking. These extrinsic influences coupled with an aging disc cell population and the stresses of everyday activities can lead to failure, which itself contributes to degenerative discs.24
Recent studies have shown that the IVD has a complex structure and mechanical properties that vary from region to region and change with age. There is evidence that the disc is capable of some regeneration. These findings plus evidence that the disc is innervated suggest that the IVD may be more than a pad that absorbs shock and maintains the spaces between the vertebral bodies. The concentration of nerves in the middle third of the disc may be sensing superoinferior compression or deformation. The circumferential arrangement of the nerve bundles about the disc and the superficial-to-deep location of the mechanoreceptors may enable the IVD to sense peripheral compression of deformation as well as alignment.25 However, a subluxation syndrome limits normal movement and function of the disc, affecting its inherent properties.
Trauma to the Cervical Spine
Nearly 4500 years ago, an Egyptian physician described a patient with a cervical spine injury as “one having a dislocation in a vertebra of his neck while he is unconscious of his two legs and his two arms, and his urine dribbles, an ailment not to be treated.”26 Although the current outlook is not so bleak, cervical spine injury continues to be a catastrophic event. There are approximately 280 spine injuries per million population each year in the United States, and approximately 10% to 30% result in spinal cord injury.27,28 The most common cause by far is motor vehicle accidents, with falls and sports-related injuries also being important traumatic events.29,30
The mortality after traumatic spinal cord injury is 47.7%, compared with 6.7% for all trauma victims. Nearly 40% of patients die before reaching a hospital and another 10% die in the hospital.31 Injuries to the upper cervical spine account for one third of cervical spine injuries, but they are responsible for 80% of the deaths of acute cervical trauma. Of those who survive, up to 70% suffer from significant neurologic deficits.32
Mechanism of Injury: General
Spinal injuries may be classified according to the mechanism of injury.33,34
Classification of Spinal Injuries
• Hyperextension fracture-dislocation
• Fracture of posterior arch of atlas
• Traumatic spondylolisthesis (hangman’s fracture)
Flexion injuries usually result from blows to the back of the head or forceful decelerations as might be experienced in motor vehicle accidents. Pure flexion trauma may result in wedge fracture of the vertebral body, without ligamentous injuries.33 These injuries are stable and are rarely associated with neurologic injuries. With more extreme trauma, the posterior column is disrupted. In severe injuries, the anterior ligament and disc space are also disrupted and bilateral facet joint dislocation results. These injuries are unstable and are associated with a high incidence of cord damage. Flexion-rotation injuries disrupt the posterior ligamentous complex and produce unilateral facet joint dislocation. These injuries tend to be stable and are not usually associated with spinal cord injury, although cervical root injury is common. The most severe of the flexion injuries is the teardrop fracture. Both columns are disrupted, and there is bilateral facet joint subluxation. The spine is unstable, and severe cord injury is seen.
Hyperextension injuries to the cervical spine result from a blow to the anterior part of the head or from an acceleration (whiplash) injury. Extension injuries are twice as common as flexion injuries, and approximately one third involve the atlantoaxial joint. These injuries are more often than not associated with cord damage. Hyperextension appears to be the most common mechanism of injury, accounting for approximately one third of all cases of cervical spine fracture.32 Hyperextension combined with compressive forces (for example, in diving) may result in fracture dislocations. The lateral vertebral masses, pedicles, and laminae are often fractured in this injury.34 Because both anterior and posterior columns are disrupted, this injury is both unstable and associated with high incidence of cord dysfunction. Violent hyperextension with fracture of the pedicles of C2 and forward movement of C2 on C3 produces the “hangman’s fracture.” The fracture is unstable, but the degree of neurologic compromise is highly variable because the bilateral pedicular fractures serve to decompress the spinal cord at the site of injury.35
Burst fractures are caused by compressive loading of the vertex of the skull in the neutral position. This type of injury is converted into a flexion or extension type with only minimal angulation of the injury force; this is relatively rare as a pure entity. Compressive forces in the lower cervical spine result in the explosion of compressed disc material into the vertebral body. Depending on the magnitude of the compression loading and associated angulating forces, the resulting injury ranges from loss of vertebral body height with relatively intact margins to complete disruption of the vertebral body. Posterior displacement of comminuted fragments may result, producing cord injury. Despite the cord injury, the spine is usually stable.34
A considerable number of fractures are misread on initial evaluation in the emergency room.36–38 The incidence of missed fractures ranges from 1% to 33%, with most series reporting 10% or more. The most common reasons for missed diagnoses are failure to take radiographs or misinterpretation of the radiograph.39 Missed injuries are often unstable, and secondary neurologic lesions are 7.5 times more common in patients who are not diagnosed at initial evaluation.37 The major factor in the development of a secondary injury is failure to immobilize the neck.40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree