Chapter 17 Cervicogenic Headache
Cervicogenic headache, tension headache, vertebrogenic migraine, convergence projection, central sensitization, central facilitation, aura
After reading this chapter you should be able to answer the following questions:
| Question 1 | What is the most common level of motion segment blockage (subluxation) found in subjects suffering “cervical headache”? |
| Question 2 | What is the proposed neuroanatomic basis of headache referred from the neck? |
| Question 3 | What cervical subluxogenic signs have been noted in headache sufferers? |
| Question 4 | Can there be a cervical component in tension-type and migraine-type headaches? |
| Question 5 | Do the results of manipulation studies automatically imply the existence of a causative cervical component to benign headaches? |
| Question 6 | Can a subluxation associated with a headache be either the cause or the effect of the headache syndrome? |
Acknowledgment of the role of the cervical spine in headache has increased since the 1990s. In 1988 a spectrum of headache subtypes that might have some kind of cervicogenic involvement was defined (Figure 17-1).1 At that time the spectrum ranged from “tension headache with neck muscle pain” through “cervicogenic headache,” defined in chiropractic terms as symptomatic head pain and cephalic dysfunction caused by subluxation of a spinal joint, to a proposed “vertebrogenic migraine.” Also in 1988, the International Headache Society (IHS) published its report on the classification of headaches.2 This classification recognized a headache subtype known as cervicogenic headache (CH). The definition of CH according to the 1988 IHS classification is shown in Box 17-1. This headache owes much of its definition to the work of Ottar Sjaastad and his colleagues,3–6 which first appeared in print in 1983.
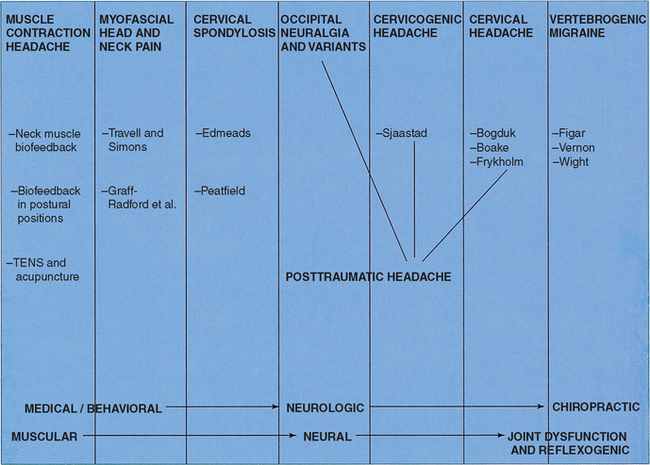
Figure 17-1 Types of headache with involvement of the cervical spine.
(From Vernon HT. Vertebrogenic headache. In: Vernon HT, editor. The upper cervical syndrome: chiropractic diagnosis and treatment. Baltimore: Williams & Wilkins; 1988. p. 152-88.)
BOX 17-1 Classification of Cervicogenic Headache Diagnostic Criteria
A. Pain localized to neck and suboccipital region. May project to forehead, orbital region, temples, vertex, or ears.
B. Pain is precipitated or aggravated by special neck movements or sustained neck postures.
C. At least one of the following occurs:
D. Radiologic examination shows at least one of the following:
From International Headache Society. Cephalalgia 1988;8 Suppl 7:1-96.
In a subsequent publication,7 this very narrow definition of CH was challenged and contrasted with the characteristic headache subtypes that chiropractic, manual medicine, osteopathic, and physiotherapeutic experts had addressed in the literature spanning the greater part of the twentieth century. The IHS version of CH was also contrasted with the headache subtypes that had been included in the clinical studies of the outcome of spinal manipulation by the same array of practitioners. In these studies of both tension-type and migraine-type headaches (definitely different from the narrow IHS features of the CH category), the results of manipulation for (presumably) some cervical spine dysfunction range from fair to excellent.
Mechanisms of Pain in Cervicogenic Headache
In 1988 a vertebrogenic model of headache was presented1 that contained four categories: (1) extrasegmental, referring to the long regional myofascial structures such as the trapezius and long extensor muscles, ligamentum nuchae, and interface between the occipitofrontalis muscle and the regional cervicothoracic structures; (2) intersegmental, referring to the three-joint complexes of C2-C3-C4 and the articulations of C0-C1-C2 with their ligaments and deep intersegmental muscles; (3) infrasegmental, referring to the nerve structures in and around the intervertebral foramina and, in the cervical spine, those near the lateral portions of the vertebrae (the sympathetic trunk; the vertebral nerve; the C2 dorsal root ganglion; the greater, lesser, and third occipital nerves; and the sensory roots of C1); and (4) intrasegmental, referring to the spinal cord and medullary dorsal horn with the nucleus subcaudalis of the trigeminal nerve.
Bogduk8 considers the possible cervical source of headache to lie in any of the structures innervated by the first three cervical nerves. Knowledge of upper cervical innervation patterns must first be considered.9
Innervation Patterns of the First Three Cervical Nerves
Localization of Somatic Tissues Innervated by the First Three Cervical Nerves
Extrasegmental
The relatively superficial, long occipitothoracic muscles in the neck include the trapezius, sternocleidomastoid, and splenius cervicis. The occipitofrontalis muscle is also an important consideration related to cranial pain. Other important extrasegmental structures include the vertebral artery (implicated in the Barré-Liéou syndrome1 and vertebrobasilar ischemic syndrome) as well as the ascending sympathetic chain and superior cervical ganglion. Older theories implicated compression or irritation of these sympathetic structures in generating cranial pain and cranial vasomotor dysregulation. These theories have fallen out of favor, having been supplanted by sensorimotor theories of pain.
Myofascial dysfunction results from macrotrauma, microtrauma, and postural strain. Trigger points with typical pain referral patterns have been charted by Travell and Simons.12 Occupational stress that produces repetitive strain can produce static overload of muscles producing local and referred pain.13
Intersegmental
These structures include the classic spinal joints and deep spinal muscles, i.e., the semispinalis occiput and cervicalis, multifidus and suboccipital muscles (posterior, lateral, and anterior). Note that there is no intervertebral disc between the C0-1 and C1-2 spinal motion segments. The suboccipital articulations include the bilateral atlantooccipital joints, the bilateral atlantoaxial joints, the atlantodental joint, joints of Luschka, and the C2-3 intervertebral disc. The suboccipital region contains a large number of specialized ligamentous structures.14
Subluxation of the C0-3 spinal motion segments is thought to be a common precipitator of cervical headaches. A number of authors have mapped local and referred pain patterns in the cervical zygapophyseal joints. Dreyfuss, Michaelson, and Fletcher15 have used provocation and anesthetic procedures to study pain patterns for the C0-1 joint. Feinstein et al.16 and others17–19 have similarly studied the C1-2 and C2-3 articulations. Anesthetic blockades have been used to identify the C2-3 Z-joint as the primary pain generator in more than 50% of a group of whiplash patients suffering from headaches.20,21
Pain patterns from trigger points in the deep intersegmental and suboccipital muscles have also been implicated.12 Tenderness in the deep suboccipital muscles is the most commonly reported finding in clinical trials.22 At least one tender point was found in 84% of a sample of tension-type and migraine headache sufferers with most exhibiting two or more. Others23 found a high prevalence of paraspinal tenderness at the C3-4 level. Bouquet et al.24 found trigger points at the C2-3 level in 24 cervicogenic headache sufferers with “enlarged C2 spinous processes,” proposed to be due to rotational misalignment at that level. Misalignment and tenderness around the transverse process of C1 were reported by Jaeger25 in a study of 11 cervicogenic headache patients.
Intrasegmental
This category involves the neural and vascular structures contained in the intervertebral environment of C1-2 and the intervertebral foramina of C2-3—specifically, the anterior and posterior rami of C1 and C2, the C2 dorsal root ganglion, as well as the C3 posterior nerve root. For an extensive review of upper cervical anatomy see Bogduk.8,26
Entrapment of the greater occipital nerve and its ganglion has long been purported to cause greater occipital neuralgia. Recent evidence by Bogduk8,26 casts more doubt on this theory, because anesthetization of the greater occipital nerve would reduce pain from any of the tissues it innervates. Irritation of the sensory fibers in the anterior ramus of C2 by inflammation or osteophytes from the C1-2 lateral joint has been reported as a cause of the uncommon “neck tongue syndrome”27 characterized by shooting pain into one side of the tongue.
Infrasegmental
Included in this category are the spinal cord and lower brainstem. Of particular importance is the spinal tract of the trigeminal nerve, which contains descending afferents from the trigeminal sensory ganglion that terminate as far caudally as C3 in the spinal nucleus of cranial nerve V. The descending tract contains three components: the pars oralis (upper), pars intermedialis, and the pars or subnucleus caudalis (lowest). These afferent fibers terminate on the same second order neurons as do the afferents from the upper three cervical roots. The second order neurons form a continuous column of cells called the “trigemino-cervical nucleus” by Bogduk and Marsland28 and the “medullary dorsal horn” by Gobel.29 This “neural anastomosis” of converging afferents is the fundamental neuroanatomical basis by which painful structures in the upper cervical region might generate referred pain to the cranium (Figure 17-2).
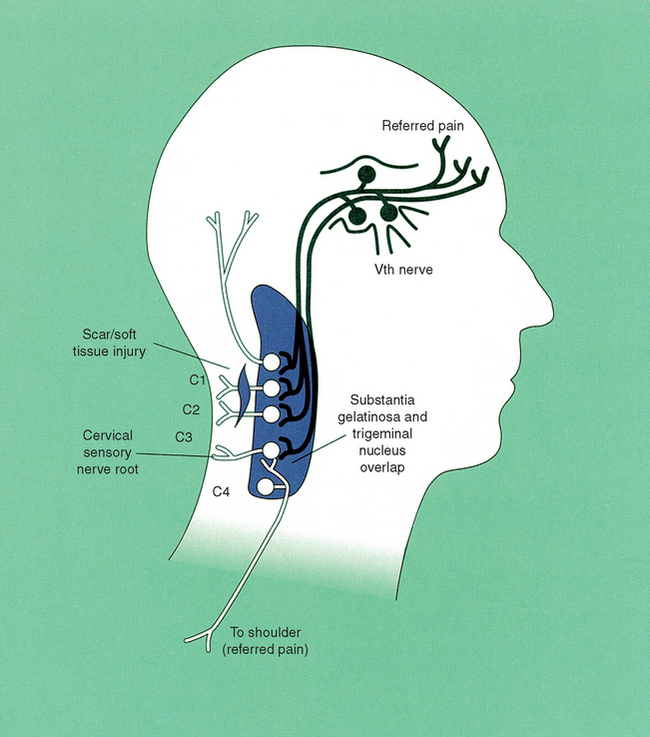
Figure 17-2 The trigeminocervical nucleus.
(From Hooshmand H. Chronic pain. London: CRC Press; 1993. p. 52.)
Only two direct mechanisms related to mechanical disturbances of the upper cervical cord have been identified. The first involves a mechanism reported by Hack and Koritzerin30 in which a ligamentous connection was noted between the rectus capitus posterior minor and the dural lining at the atlantooccipital junction. In a small number of reported cases, surgical ligation of this ligament has resulted in improvement in headache patients. The second mechanism involves a herniation of the C2-3 intervertebral disc. Reported in 1950 by Eldridge and Choh-Luh,31 this is thought to be relatively rare. Recently disc fusion surgery with the use of discogram imaging has resurrected this idea.
A significant role played by the spinal cord in cervicogenic headache lies in the previously described phenomenon of afferent convergence of the upper cervical and trigeminal systems.32 This mechanism explains the referred pain to the cranium resulting from upper cervical deep-tissue pain. It should be remembered that the same convergence phenomenon explains why posterior intracranial pathologies result in referred upper cervical pain. This may be one of the mechanisms involved in the creation of upper cervical pain and myofascial dysfunction in migraine. Painful and inflamed posterior cranial vessels can refer pain to the suboccipital region contributing to diagnostic confusion.
Implications for Headache of Cervical Origin
Central sensitization provides a mechanism to explain the clinical phenomena seen so regularly in headache sufferers of (1) persistent somatic pain; (2) pain referred from the cervical spine or posterior occipital region into the frontoorbital regions and perceived as “headache” when it is actually referred neck pain; (3) tender hyperalgesic muscle zones (“trigger points”), which often expand as the headache pain increases; (4) muscular tension in the deep suboccipital, superficial occipital, and craniofacial muscles, which has for many years been thought to be the sole basis of muscle contraction or tension-type headache.33 Conversely, the mechanism of “convergence-sensitization-projection” also serves to explain how pain arising from intracranial structures such as posterior cerebellar tumors or the intracerebral blood vessels (proposed by Moskowitz34 in the case of migraine) may be referred to the posterior occipital and suboccipital regions, masquerading as cervical pain.
In summary, the trigeminocervical nucleus and the extensive afferent convergence from numerous craniocervical peripheral tissues onto these second-order neurons (a phenomenon called convergence-projection)35 serves to explain the neuroanatomic basis of headache referred from the neck. The neurophysiologic basis of such pain referral, particularly from inflammatory pain arising from the posterior suboccipital muscles and joints, is explained by the phenomenon of “central sensitization” and the neuroplastic changes that these second-order neurons undergo in response to prolonged peripheral deep somatic pain. These are the mechanisms thought for many years by chiropractors to arise from the subluxation or dysfunction state of the vertebral motion segment. Certainly this is consistent with the older model of “central facilitation” proposed by Korr et al.36 and adhered to by several generations of chiropractors.
Cervicogenic Dysfunction in Headache
A 1992 report by Vernon et al.22 on cervicogenic dysfunction in muscle contraction (that is, “tension-type headache” [TTH]) and common migraine (that is, “migraine without aura” [MWA]) defined the components of cervicogenic dysfunction, and the literature up to 1988 was reviewed in defense of the notion of a broad, highly prevalent basis of cervicogenic dysfunction in headache. A 1991 report7 also addressed how high prevalence of cervicogenic dysfunction in these types of headaches argued against the position adopted by the IHS (based principally on the work of Sjaastad and his colleagues3–6) that “cervicogenic headache” was a narrowly defined, infrequently encountered form of headache. This controversy was again addressed by Vernon in 1999.37
Components of cervicogenic dysfunction:
1. Hypomobility; variously termed subluxation, joint blockage, segmental dysfunction, fixation (See Chapter 1.)
2. Tender points in the soft tissues; local areas of hypersensitivity that produce a pain response to light pressure
3. Trigger points; palpable nodules in muscles that refer pain in consistent patterns
4. Radiographic findings of misalignment and dynamic intersegmental abnormality
5. Static segmental misalignment on palpation
6. Static malposition of the head and neck (specifically, anterior carriage of the head and low rounded shoulders)
Hypomobility
In 1985 and 1986, Jull38,39 reported on both the reliability of upper cervical joint motion palpation and its use in headache subjects to determine the lesioned segment. The comparison between headache and nonheadache subjects39 showed dysfunction at C0-1, C1-2, and C2-3 in 60%, 40%, and 55% of headache subjects, compared with 5%, 12%, and 22%, respectively, in controls. These palpatory findings were confirmed by Jull, Bogduk, and Marsland40 and Dwyer et al.41 using diagnostic anesthetic blocks as the gold standard.
In the 1992 report by Vernon et al.,22 three motion palpation procedures described by Fligg42 were used. They were A-P glide, rotation, and lateral flexion. A major blockage in any of these three procedures on either side at C0-3 was indicative of segmental fixation. It was reported that only 16% in each group had a fixation at only one level. In the tension-type group, 54% had fixations at two levels and 30% at all three; for migraine subjects these figures were 42% and 42%, respectively. In both groups, 84% had a fixation in at least two of three upper cervical segments.
In 1993 Watson and Trott43 used multiple outcomes (others are discussed later) to assess cervical headache subjects. They reported on the reliability of posterior-to-anterior glide palpation in 12 of the subjects examined on two occasions by the same examiner. (Kappa values ranged from 0.67 to 1.0 depending on the segment.) They also included as positive signs of joint dysfunction the presence of tenderness and muscle stiffness. (See later discussion.) When all three signs—fixation, tenderness, and palpatory stiffness—were included, far more positive findings were found in headache subjects (N = 30) than in controls (N = 30). The most prevalent level was C0-1.
In 1997 Jull et al.44 reported high rates of agreement between several pairs of examiners in their abilities to detect the presence or absence of treatable upper cervical dysfunction. Agreement levels were somewhat lower on the exact segment of dysfunction but still good at 70% overall. The highest frequency of joint dysfunction was found at the C1-2 segment. The sensitivity of motion palpation was reported by Jull et al.44 as 100%. They also reported high levels of agreement between motion palpation findings that were obtained by examiners without pain cues and from headache subjects’ subsequent reports of pain during each procedure at each cervical motion segment. This established validity of motion palpation without the subject providing pain-related feedback. Much higher levels of significant joint dysfunction were found in the upper cervical segments of cervical headache subjects versus controls in this study. Jensen, Nielson, and Vosmer45 reported findings of hypomobility before and after treatment with manipulation compared to the use of cold packs, in a clinical trial of 19 posttraumatic headache patients. The most frequently blocked segment was C1-2. Fourteen of the subjects had at least one level of joint blockage in the upper cervical and upper thoracic region, whereas four had blockage only in the upper cervical region for a total of 18 out of 19 patients with upper cervical hypomobility.
Vernon, Steiman, and Hagino22 found that 54% of tension-type headaches had hypomobility at two upper cervical segments, whereas 30% had all three levels affected at least unilaterally. They also found that migraine headache sufferers had 42% and 42%, respectively. At least 84% in both groups had at least two upper cervical segments exhibiting hypomobility.
In a posttraumatic headache study46 that compared headache subjects with an age- and gender-matched control group, at least one segment in the headache group demonstrated marked hypomobility in the upper cervical spine. Much greater joint dysfunction was noted between C0-3 in the headache group as compared with the control group.
In a cervical migraine study, Stodolny and Chmeilewski47 reported that all 31 of the headache patients had significant joint dysfunction at C0-1 on manual palpation. More than 80% of these subjects had at least two cervical motion segments demonstrating joint dysfunction, remarkably consistent with the findings of Vernon, Steiman, and Hagino22 in their study of tension-type headaches and migraine subjects.
Craniovertebral Tender Points
Tenderness to palpation of the skin48 and deep tissues of the craniovertebral and paraspinal region is the most commonly reported sign of headache of cervical origin. Virtually every relevant author has reported on the subject, from Lewit,49 who reported on “pain over the posterior arch of atlas,” to Sachse et al.,50 who reported similar suboccipital and scapular tenderness, to Graff-Radford et al.51 and Jaeger,25 who have reported on the numerous cervical tender points that serve to perpetuate myofascial head pain, to Sjaastad et al.,3,4 who reported the high prevalence of tenderness at C2-3.
A 1992 study37 reported on the prevalence of six standard craniocervical tender points in these headache groups:
Table 17-1 lists the studies of manual tenderness assessment in neck pain and headaches.
Table 17-1 Studies of Manual Tenderness Assessment in Neck Pain and Headaches
| Author(s) | Findings | Location |
|---|---|---|
| Lebbink, Spierings, and Messinger | Neck muscle soreness, stiffness, and prior neck injury more common in 164 headache sufferers than in 108 controls | Neck muscles |
| Jensen et al. | Studied 14 muscles sites bilaterally in normals; used Langemark et al. method of scoring; norms reported; older subjects had lower TTS values, females had higher TTS scores | Cranial and large neck muscles |
| Jensen et al. | TTS scores in TTH and migraine headache sufferers compared; TTHs had lower overall scores; TTHs with headache that day had higher TTS than matched non-HA group | Cranial and large neck muscles |
| Jensen, Nielsen, and Vosmar | In 19 PTHAs, 42% had tenderness at C2-C3, 89% at C3-C4, and 63% at C4-C5 | Neck paraspinal muscles |
| Hatch et al. | HA subjects had at least one tender muscle more often than controls; TTS in HAs greater than controls; EMG findings not correlated to tenderness | Four cranial muscles |
| Two posterior cervical muscles | ||
| Watson and Trott | PTHAs had more tenderness findings than controls, particularly in upper cervical spine | Neck paraspinal muscles |
| Mercer, Marcus, and Nash | HA subjects had higher values of tenderness than controls | Neck paraspinal muscles |
| Levoska, Keinanen-Kiukaannierni, and Bloigu | Test-retested correlation of manual palpation of scapular muscles was high; interrater reliability only fair | Scapular muscles |
| Levoska et al. | Neck pain sufferers had higher number of tender points than controls | Neck paraspinal muscles |
| Hubka and Phelan | Interrater reliability of segmental TTS scores was highly correlated (Kappa, 0.68) | Neck paraspinal muscles |
| Sandmark and Nisell | Cervical tenderness was most sensitive (82%) and specific (79%) for neck pain patient discrimination | Neck paraspinal muscles |
| Nilsson | TTS scores in neck pain patients; high interrater reliability | Neck paraspinal muscles |
| Sandrini et al. | Mean TTS scores higher in ETTH and CTTH subjects than controls | Trapezius |
| Persson and Carlsson | TTS scores higher in CH vs. controls | Suboccipital, neck paraspinal, and scapular muscles |
| Stolk-Hornsveld et al. | Segmental tenderness on passive motion at C1-C4 higher in CH vs. other headache types; good interrater reliability | Suboccipital and neck paraspinal muscles |
TTS, Total tenderness score; TTH, tension-type headache; HA, headache; PTHA, posttraumatic headache; EMG, electromyogram; ETTH, episodic tension-type headache; CTTH, chronic tension-type headache; CH, cervicogenic headache.
From Redwood D, Cleveland CS. Fundamentals of chiropractic. St. Louis: Mosby; 2003. p. 515-6.
In the 1992 report, the pressure algometer37 was used to verify true tenderness in cervical tender points in tension-type and migraine-without-aura sufferers. This type of assessment has been used with great success by fibromyalgia52 and headache53 researchers. Table 17-2 summarizes studies of pressure algometry in neck pain and headache. In fact, the 1988 IHS classification2 includes the presence or absence of pericranial tender points as part of the subclassification of tension-type headaches. (See Box 17-1.) A recent report by Kanieki54 has found tender points in migraineurs during attacks.
Table 17-2 Studies of Pressure Algometry in Neck Pain and Headache
| Author(s) | Findings | Location |
|---|---|---|
| Reeves, Jaeger, and Graff-Radford | High correlation coefficients for intraexaminer and interexaminer reliability; average value for C0-C1, 3.0 kg/cm2; for trapezius, 3.5 kg/cm2 | Occipital and suboccipital |
| Jensen et al. | Highly consistent values bilaterally and over 3-week interval in normals | Temporalis muscle |
| Drummond | High intraexaminer reliability; HA subjects had lower algometer values than normals; no difference between TH and migraine HA | Scalp and upper cervical muscles |
| List, Helkimo, and Falk | High intraexaminer reliability; algometry scores highly correlated to manual palpation findings; TMJ pain subjects had lower values than normals | Temporalis and suboccipital |
| Langemark et al. | Temporalis algometry negatively correlated to headache intensity and to TTS on manual palpation; high correlation between temporal and occipital sites | Cranial muscles |
| Takala | High intrarater and interrater reliability in normal subjects; women had lower algometry values than men; lower values in subgroup with minor neck pain and HA | Scapular muscles |
| Hogeweg et al. | Good reliability to normals; cervical points have lower algometry values than lumbar points | Spinal muscles |
| Bovim | Lower algometry values in cervicogenic HA group vs. migraine, TTH, and controls; CH group had lower values in posterior cranial area and on the affected side | Cranial and suboccipital muscles |
| Chung, Un, and Kim | Electronic pressure algometer showed good reliability and test-retest consistency in normals | TMJ and neck muscles |
| Jensen et al. | Algometry values lower in TTH vs. controls | Cranial muscles |
| Kosek, Ekholm, and Nordema | Algometry in normals showed good 1-week consistency; lower values in upper part of body | Whole body |
| Levoska, Keinanen-Kiukaanniemi, and Bloigu; Levoska | Reliability high in neck pain and normals; pain group had lower values | Scapular muscles |
| Massotta et al. | PPT values significantly lower in ETTH vs. controls | Temporalis |
| Sandrini et al. | PPT values significantly lower in ETTH and CTTH vs. controls | Frontalis and trapezius |
| Stolk-Hornsveld et al. | High levels of interrater reliability; sensitivity and specificity for CH vs. controls, 82% and 62% | Suboccipital and neck paraspinal muscles |
| Bendtsen et al. | Reported on an electronic finger pressure pad for palpating tenderness; high levels of interexaminer reliability | Cranial muscles |
HA, Headache; TTH, tension-type headache; TMJ, temporomandibular joint; TTS, total tenderness score; CH, cervicogenic headache; PPT, pressure pain threshold; ETTH, episodic tension-type headache; CTTH, chronic tension-type headache.
From Redwood D, Cleveland CS. Fundamentals of chiropractic. St. Louis: Mosby; 2003. p. 517.
The findings of Watson and Trott43 in which detection of joint dysfunction in headache (HA) subjects and non-HA subjects included pressure palpation for tenderness were described above. Again, this procedure was found to have good intraexaminer reliability and was found to distinguish HA subjects from non-HA subjects with higher prevalence of findings in the HA subjects.
Finally, tenderness to palpation has been used as one of the cardinal signs by Jull, Bogduk, and colleagues38–41 to locate the level of zygapophyseal joint dysfunction potentially responsible for neck pain and headache. These findings correlate very well with the signs of joint hypomobility previously discussed. This combination of tenderness and hypomobility (as with Watson and Trott43) correlates very highly with joint blockades used as a gold standard for diagnosis.
A finding associated with muscle tenderness is increased muscle stiffness. In 1992 Vernon and Gitelman55 reported on a single case of bilateral tension-type headache with cervical dysfunction. Pressure algometry showed clinically significant tenderness bilaterally in the suboccipital region. Muscle stiffness of the mid-cervical paraspinal and trapezius muscles was measured using Fischer’s tissue compliance meter.56 Higher than expected values were found in both muscle sites.
Sakai et al.57 used a computerized compliance meter in a comparison of 37 tension-type headache subjects and 63 normal individuals. In 65% of HA subjects there was significantly increased trapezius stiffness, and the overall mean values (756 + 121 versus 538 + 89) significantly distinguished headache from control subjects. An orally administered muscle relaxant greatly reduced this increased stiffness in headache subjects, implying that active tension contributed to the stiffness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








