CHAPTER 57 Cervical Radicular Pain: An Algorithmic Methodology
Employing an algorithmic methodology for the evaluation of cervical radicular pain has numerous clinical benefits. It simultaneously combines elegant simplicity with diagnostic meticulousness. It helps generate a comprehensive differential diagnosis in complex conditions and prioritizes these diagnoses. To use such an algorithm effectively, however, it is mandated that the clinician gain the necessary fund of knowledge behind the various branches in the decision tree so that each patient is treated individually to optimize functional outcome. The old adage that ‘a smart man knows the rules, but a wise man knows the exceptions’ holds true. The evaluation of cervical radicular pain employs an overall assessment of historical and examination features, electrodiagnostic testing, diagnostic imaging, and diagnostic anesthetization of a suspected pain generator. A basic tenet underpinning the use of the above tools is that an accurate diagnosis allows for specific treatment and therefore better outcomes.
ANATOMY
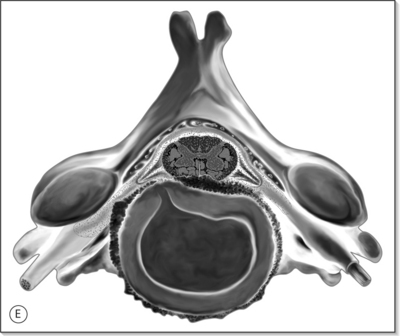
Anatomy of the cervical spine is covered in more detail in Chapter 46 by Russell Gilchrist. A brief discussion of the relevant cervical spine anatomy is discussed here to provide the reader with some background information. The cervical spine is the most mobile segment of the axial skeleton, and serves to support and move the head, and protects the neural elements within. There are seven cervical vertebrae which can be divided into upper (C1–2 or atlantoaxial joint) and lower segments (C3–C7). The C1 vertebrae (atlas) and the C2 (axis) are different in function than the lower segments. The atlas is a ring-like structure without a vertebral body. The lateral masses of C1 articulate with the occipital condyles above and the axis below. The axis has a vertebral body and an odontoid process which is the congenitally fused body of the atlas. The C1–2 joint allows for 58% of all cervical rotation, or 45° of rotation in either direction.1 The atlanto-occipital articulation permits 10° of flexion and 25° of extension.2 The C2–3 segment represents a mechanically transitional area where flexion, extension, and lateral bending become increasingly permitted, and is the most common zygapophyseal joint associated with whiplash-related cervicogenic headaches. The lower cervical vertebrae (C3–C7) articulate via the intervertebral discs anteriorly and the cervical zygapophyseal joints posteriorly. The zygapophyseal joint has a 45° orientation relative to the horizontal plane which allows for ipsilateral bending and rotation. Lateral bending occurs primarily at C3–4 and C4–5. The C3–C7 segments have articulations known as uncovertebral joints, or joints of Luschka, along the posterolateral border of the intervertebral disc, and are in the anteromedial portion of the intervertebral foramen. These articulations are not true synovial joints, but can hypertrophy with associated disc degeneration and result in a narrowing of the intervertebral foramen and potential neural compromise.
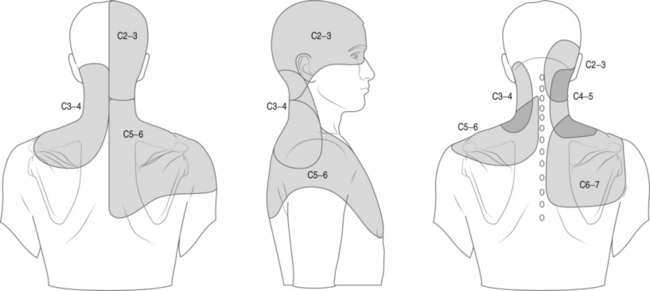
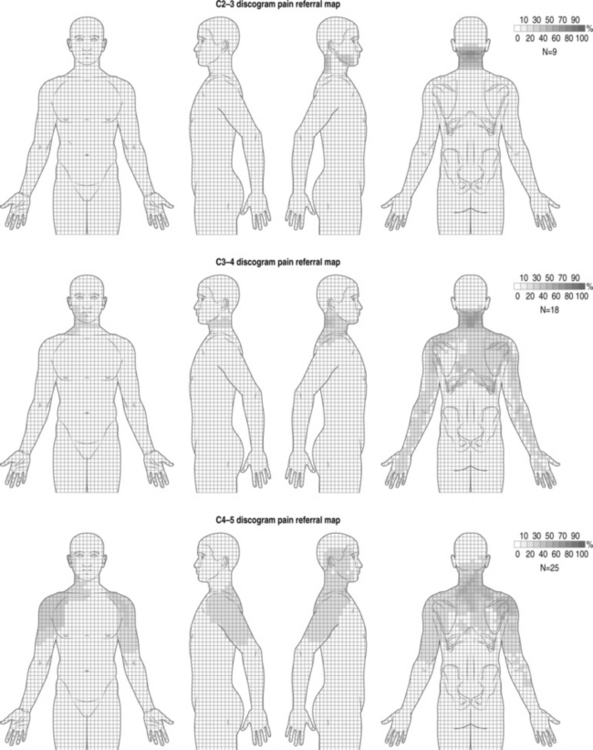
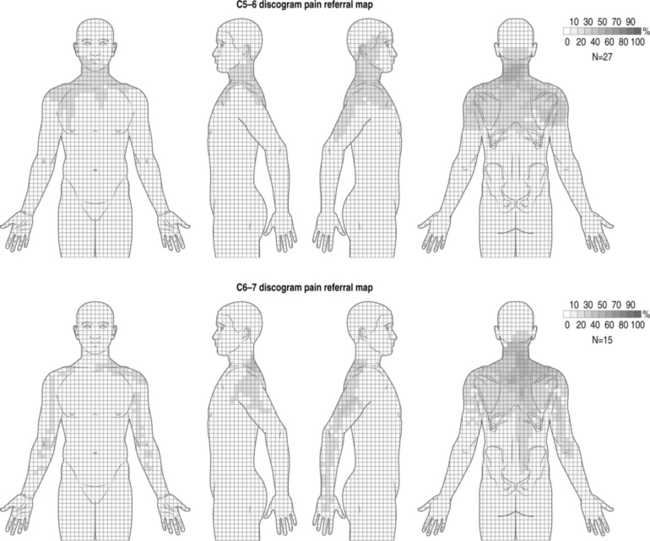
Cervical facet syndrome can often mimic radicular pain and refer symptoms to the limb. The differentiation between facetogenic or radicular symptoms is facilitated by an awareness of two facts. Firstly, the axial neck symptoms are often more severe than extremity symptoms (Fig. 57.1).3–7 Secondly, the cervical facet joint does not refer symptoms distal to the elbow. Please see Figure 57.2, which illustrates pain referral patterns of the cervical zygapophyseal joints. The cervical zygapophyseal joints are innervated by medial branches of dorsal rami from C3–4 to C6–7 that run above and below a given level. The superficial medial branch of the C3 dorsal ramus, or the third occipital nerve, innervates the C2–3 joint.8 The ventral rami of C1 innervate the atlanto-occipital joint, and C2 rami innervate the atlantoaxial joint. The C1 nerve root contains motor fibers only and emerges from the spinal canal at the atlanto-occipital junction. The cervical intervertebral discs receive innervation in the outer third of the anulus. The anterior portion of the disc is innervated via the vertebral nerve (derived from the gray rami communicans of the sympathetic trunk at midcervical levels and the branches of the stellate ganglion at lower levels). The posterior and lateral portions of the disc receive innervation via the sinuvertebral nerve (recurrent nerve of Luschka, derived from a branch of the vertebral nerve and ventral ramus at each level). The sinuvertebral nerve innervates the disc and the disc above a given segment, the pedicle, posterior longitudinal ligament, posterior vertebral periosteum, epidural veins, and dorsal dura mater. The cervical spinal cord is approximately 10 mm in diameter, and the vertebral canal averages 17 mm in diameter. The cervical central canal is almost twice as wide laterally as it is in the anteroposterior direction and is widest at C3–5. In contrast, the thoracic spinal canal is more round and narrow. The radicular complex of ganglia, spinal nerve root, and surrounding sheath account for 20–35% of the cross-sectional area of the foramen.9 The cervical foramen are widest at C2–3 and progressively decrease in size to the C6–7 level. This diminution in size, combined with the increased segmental motion in the lower cervical spine, likely affects the incidence of degenerative disc disease in the cervical spine and resultant cervical radiculopathy. Cervical radiculopathy secondary to spondylosis most commonly affects the seventh (60–70%), sixth (16–25%), eighth (4–10%), and fifth (2%) cervical spinal nerves.10–12 In younger patients, cervical radiculopathy is more commonly secondary to trauma or disc herniation whereas in older individuals it is often secondary to uncovertebral hypertrophy with resultant foramenal stenosis.
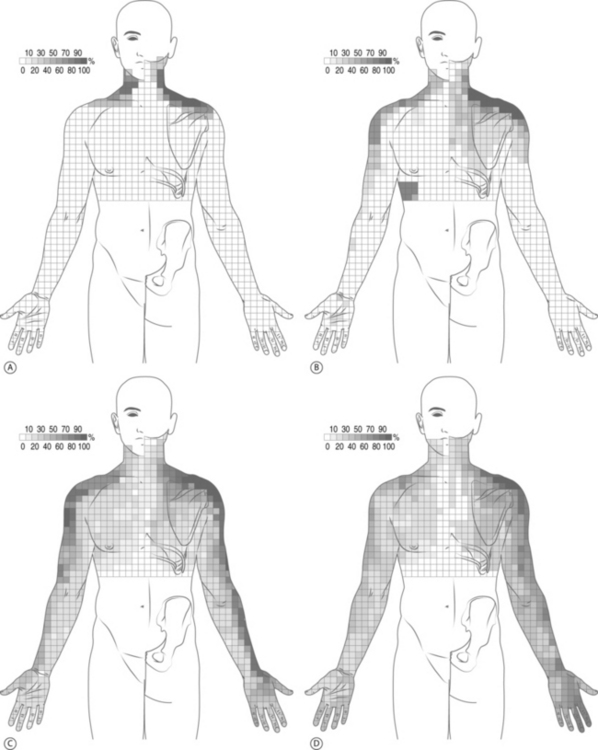
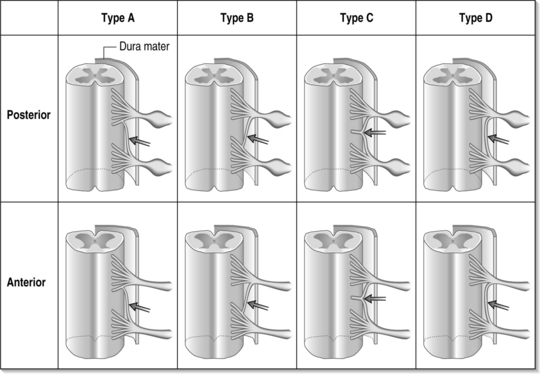
Determining the symptomatic nerve root often begins with asking the patient to describe the areas of pain. Cervical dermatomal charts were first studied in the late nineteenth century by Sherrington;13,14 however, patients with radicular symptoms may have symptoms other than the dermatome implicated by imaging studies. A dynatome is a pattern of referred symptoms, and cervical dynatomes for cervical nerve roots C4 through C8 have been studied. Slipman et al.’s dynatomal mapping15 derived cervical spinal nerve root stimulation referral patterns by mechanically stimulating a given spinal nerve and asking patients to delineate areas of symptom referral (Fig. 57.3). It has been demonstrated that there is overlap between dermatomal and dynatomal maps; however, symptoms can be commonly referred outside of a given dermatome. This may be secondary to the high incidence of intrathecal anastomoses between cervical spinal nerve roots, as high as 61% between the dorsal nerve roots and 10% for the ventral roots.16 Please see Figure 57.4, which demonstrates different types of intrathecal anastomoses noted by Moriishi et al. This high incidence of anastomoses in the dorsal roots can result in atypical referral patterns. For example, the little finger is classically involved in patients with C8 radicular pain, however 30% of C6 nerve root stimulations can produce little finger symptoms. Also noted was that the index finger, classically involved in patients with C6 or C7 radicular pain, can be involved in 24% of C8 nerve root stimulations.15 Other possible factors that may confound the evaluation include somatic referral of axial pain which can often mimic radicular pain, conjoined nerve roots, multilevel neural compression, concomitant musculoskeletal diagnoses, concomitant peripheral entrapment syndromes, and psychological distress due to chronic pain.
PATHOPHYSIOLOGY OF RADICULAR PAIN
The degenerative cascade that occurs with age likely begins with degeneration of the cervical intervertebral disc. Viscoelastic properties of the disc change trend toward desiccation as age-related alterations in the chemical composition of the nucleus pulposus and anulus fibrosus occur. The disc loses height and bulges posteriorly into the canal by a hoop tension effect. The loss of disc height anteriorly results in infolding of the ligamentum flavum and facet joint capsule posteriorly. This results in a decrease in both the size of the central canal and neural foramen. Osteophytes form around the disc margins, uncovertebral articulations, and the zygapophyseal joints. The uncovertebral articulations, which represent a degenerative cleft on the posterolateral and foramenal region of the vertebral body, hypertrophy, which results in foramenal narrowing. The resultant decrease in canal size can result in compromise of the spinal nerve roots or spinal cord. Mechanical compression of the nerve root may lead to motor weakness or sensory deficits. Radicular pain is generally thought to be secondary to an inflammatory response and subsequent involvement of the nerve root. Increased permeability within intraneural blood vessels result in edema of the nerve root. The dorsal root ganglion has been implicated in the pathogenesis of radicular pain and radiculopathy. Chronic edema and fibrosis within the nerve root can increase the sensitivity of the nerve root to pain by lowering the excitation threshold.17
Much of the research investigating the biochemical pathophysiology of radiculopathy has been performed in the lumbar spine. The relative abundance of studies investigating lumbar radiculopathy reflects its higher clinical incidence compared to cervical radiculopathy. Given the relative paucity of studies examining the biochemical mediators involved in cervical radicular pain, one needs to make a leap of faith to conclude that parallels exist with the lumbar spine. While these parallels may exist for cervical radicular pain due to disc protrusions, it may not be the case in patients with cervical radiculopathy due to foramenal narrowing due to uncovertebral hypertrophy, or whiplash-induced post-traumatic radicular pain. A discussion of some of the salient research performed in the lumbar spine is included below. It may be reasonable to conclude that many of the same biochemical pathways are involved in some etiologies of cervical radicular pain. Biochemical mediators of pain released from the cell bodies of the sensory neurons located in the dorsal root ganglion lower the impulse propagation threshold and biochemical mediators released from the intervertebral disc activate the arachidonic acid cascade. There have been numerous studies demonstrating a biochemical inflammatory process mediating pain in radiculopathy. Saal et al. found elevated phospholipase A 2 in disc material obtained from patients treated surgically for radiculopathy.18 Phospholipase A2 is the rate limiting step in the arachidonic acid pathway, which subsequently generates prostaglandins and leukotrienes.19 Prostaglandin E2 has been shown to be elevated in disc material at the time of surgery, and it has been shown to play a role in sensitizing nociceptors to bradykinins.20 Additionally, phospholipase A 2 has been shown to be neurotoxic.21 In work by Takahashi and co-investigators, disc material obtained at surgery in humans was assayed and cultured. They studied disc protrusion, extrusion, and sequestration. They found that with disc protrusion there was an increased number of chondrocytes. In contrast, with extrusion and sequestration, there were an increased number of histiocytes, fibroblasts, and endothelial cells with relatively few chondrocytes.22 Cytokines are expressed by these cells and there is no apparent difference in the groups of cytokines produced when comparing disc protrusion, extrusion, or sequestration. Betamethasone added to the cell cultures of disc materials inhibited cytokine and prostaglandin E2 production. In a study by Doita et al., disc samples obtained from surgical patients with an extrusion or sequestration showed increased granulation tissue and increased monocytes expressing interleukin-1.23 Interleukin-1 is known to stimulate inflammatory mediators and proteolytic enzymes including plasminogen activator, collagenase, and stromelysis. Kang and investigators assayed and compared disc tissue from patients being operated on for scoliosis and a herniated disc. They observed elevated levels of matrix metalloproteinase, nitric oxide, prostaglandin E2, and interleukin-6 in the herniated disc group. Concentrations of interleukin-1, tumor necrosis factor and interleukin-1 receptor antagonist protein were not appreciably increased or different in either group.24 Matrix metalloproteinase-3, inhibitor of matrix metalloproteinase-1, calcitonin gene-related peptide, vasoactive intestinal peptide, and substance P have all been associated with disc degeneration.25–29 It has been postulated that these inflammatory mediators play a role in lumbar radicular pain. Immune responses to nucleus pulposus in the vascular space has been theorized to be a mechanism of chronic inflammation. Work by Gertzbein demonstrated significant elevation in the lymphocyte transformation test, a measure of the cellular immune response.30 IgM titers were also increased in 2 of 3 patients with discogenic back pain or sciatica.31 These changes in the biochemical milieu contribute to the inflammatory response and subsequent hypersensitivity of the nerve root to stimulation.32–36
Cervical radicular pain can occur in the absence of significant degenerative changes in patients with a history of trauma. Whiplash events can injure many structures in the spine including the muscles and ligaments, intervertebral disc, bony structures, zygapophyseal joints, and cervical spinal nerve roots. There is a paucity of literature focusing on nerve root injury secondary to a whiplash event. Keith in 1986 described an observational case series of 14 patients with persistent post-whiplash symptoms which included unilateral occipital and suboccipital pain, tenderness, and hypoesthesia. The author postulated a causative injury to the second cervical ganglion and nerve root.37 Örtengren et al. confirmed the presence of gangliar and radicular injury in whiplash in an experimental pig model. Using pigs in simulated flexion–extension whiplash events, Örtengren was able to show increased gangliar cytoplasmic uptake of Evans blue, a dye usually excluded from this intracellular region in healthy ganglion cells. Increased dye uptake was found most frequently in the C4–C7 spinal nerves, and was not found in a control group of pigs that underwent a sham whiplash simulation. The author postulated that transient elevations in intraforaminal pressure are the cause of the neural injury. These pressure increases are due to two factors: mechanical load on the foramen itself due to discordant supra- and subjacent vertebral motions, as well as spikes in intraforaminal venous pressure due to nearby alterations in pressure in the epidural venous plexa.38 Exact values for such pressures in humans have yet to be elucidated, if indeed this mechanism is the cause of spinal nerve injury in whiplash.
Studies have described neuronal malfunction in response to axonal injury, leading some to consider distal axonal damage as a possible source of dorsal root ganglion injury in whiplash.39 The underlying cause of neural damage in whiplash remains unresolved. Symptomatology suggestive of radicular injury, including radiating pain, is reported widely in the literature. Objective dermatomal hypoesthesia has been a relatively rare finding, however.39,40 Cervical nerve root injury can occur in the absence of MRI correlate.41 In a study by Slipman et al., patients with clinical evidence of radicular pain, as demonstrated by a positive Spurling’s sign or a positive upper extremity root tension sign in combination with increased pain during extension or ipsilateral rotation, without any corroborative pathology with advanced cervical imaging, underwent diagnostic selective nerve root blocks to identify the pain generating spinal nerve. Subsequent therapeutic selective nerve root blocks were given with only 14% of patients achieving a good or excellent outcome. A transient steroid effect was noted in 59% of patients. The authors concluded that selective nerve root blocks using glucocorticoid are not an effective treatment for whiplash-induced cervical radicular pain.42 The poor outcomes reported suggest an underlying etiology that does not include an inflammatory response, thereby illustrating that there is likely a multitude of mechanisms by which cervical radicular pain can develop and be perpetuated.
CLINICAL ASSESSMENT
History
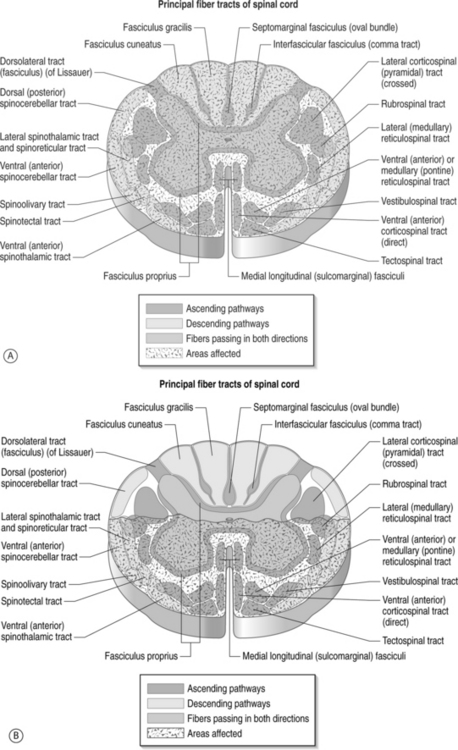
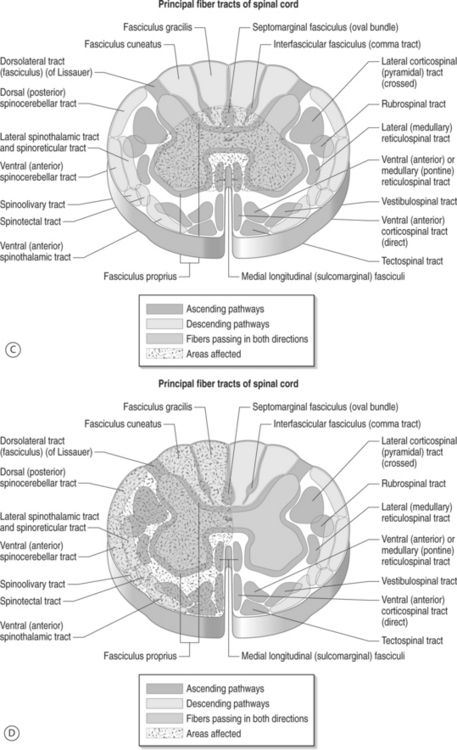
Symptoms related to the cervical spine can be broadly categorized as predominantly axial pain, extremity pain, or myelopathy. Combinations of the above are often present, but making this initial categorization helps with generating a differential diagnosis and further instituting treatment. Patients with cervical radicular pain will often complain of symptoms in the upper limb which are worse than axial neck pain, and symptoms are typically exacerbated with sustained neck position, neck extension, neck rotation, and ipsilateral limb abduction with elbow extension. Symptoms are often alleviated by positional shifting, maintaining a neutral posture to the spine, avoidance of neck rotation, and overhead abduction with elbow flexion, also known as Bakody’s sign (Fig. 57.5). Conditions such as cervical discogenic pain/cervical internal disc disruption syndrome and cervical facet joint syndrome cause a predominance of axial neck pain but can refer symptoms to the limb without the compression or involvement of the cervical spinal nerve root. In contrast to cervical facet-mediated pain, the cervical disc can refer symptoms into the extremity that is distal to the elbow (Fig. 57.6).43 This mechanism is termed somatic referral, which was previously known as sclerotomal referral. This referred pain may be via central mechanisms. Patients with this syndrome will often describe axial pain to be greater than limb pain. In contrast, radicular pain will more commonly have limb pain greater than axial pain. Identifying whether the referred symptoms are secondary to radicular pain versus somatic referral can often be ascertained through clinical assessment. Cervical myelopathy can occur simultaneously with radiculopathy (myeloradiculopathy). The identification of myelopathy is critical because a specific subset of patients will require surgery and their outcomes are improved when this decision is not delayed. Patients with myelopathy can present with different patterns in which upper motor neuron symptoms predominate secondary to injury to the spinal cord (Fig. 57.7): (1) transverse lesion syndrome, in which the corticospinal, spinothalamic, and posterior cord tracts were involved with almost equal severity and which is associated with the longest duration of symptoms, suggesting that this category may be an end stage of the disease; (2) anterior cord or motor system syndrome, in which corticospinal tracts and anterior horn cells are involved, resulting in motor weakness and spasticity with relative preservation of sensation – this is usually due to an anterior spinal artery infarction, which is a single artery located in the anterior median fissure and supplies the anterior two-thirds of the spinal cord; (3) central cord syndrome, in which motor and sensory deficits affected the upper extremities more severely than the lower extremities – this is usually seen in syringomyelia or hydromyelia, and trauma; (4) Brown-Séquard syndrome, which consists of ipsilateral motor deficits and ipsilateral loss of vibration and proprioception, with contralateral sensory deficits to pain and temperature – this is most commonly seen after trauma;. and (5) myeloradiculopathy or brachialgia and cord syndrome, which consists of radicular pain in the upper extremity along with motor and/or sensory long-tract signs.
An algorithmic approach
Treatment of a patient’s pain complaints should employ a diagnosis-specific approach. Obtaining a specific diagnosis will ensure that the best treatment is instituted for a given complaint. Treatment of cervical radicular pain involves a spectrum of treatment options which include education for the patient, activity and postural and worksite modifications, therapeutic exercise, adjunctive modalities, cervical traction, pharmacologic measures, fluoroscopically guided injection procedures, and surgical decompression. Treatment should be individualized and goal oriented to ensure successful patient outcomes. Details of the different treatment options are discussed in more depth in Chapters 59–61.
Atraumatic spondylotic radicular pain
Cervical degenerative disc disease is exceedingly common, and its incidence increases with aging. Over half of the middle-aged population has radiologic or pathologic evidence of cervical spondylosis.44,45 Only a minority of these individuals become clinically symptomatic and develop axial neck pain, cervical radicular pain, or cervical myelopathy. The etiology of axial neck pain is often secondary to either musculoskeletal sprain and strain, discogenic pain secondary to cervical internal disc disruption syndrome, or cervical facet syndrome. These disorders typically have a predominance of axial pain with relatively minor or no extremity symptoms. In contrast, patients with predominance of scapular and extremity symptoms more commonly have involvement of the cervical spinal nerve root or spinal cord itself. When determining the symptomatic spinal nerve root in patients with atraumatic cervical radicular pain, the clinician can often employ parsimony and thus one or two predominant pain generators can often be established.
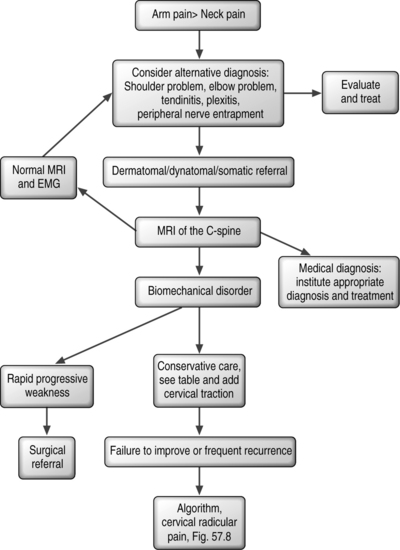
Many patients with atraumatic spondylotic cervical radicular pain present with typical historical and examination features, and corroborative imaging. For these patients, establishing a pain generator and an algorithm for treatment is more straightforward (Fig. 57.8). In the presence of severe weakness and/or progressive weakness, surgical decompression is considered the intervention of choice. Determining if the weakness is progressive requires skillful neurological testing, and a detailed understanding of the Medical Research Council 0 to 5 scale manual muscle testing technique. Additionally, the patient should be asked as to whether they are aware of progressive weakness and of any functional deficits relating to the affected limb. In the absence of progressive weakness, conservative options such as pharmacologic measures, physical therapy, and injections targeted toward the affected spinal nerve root can be used. If conservative measures fail to bring adequate pain relief and restoration of function, surgical options of percutaneous or open decompression may be considered.
Traumatic spondylotic cervical radicular pain
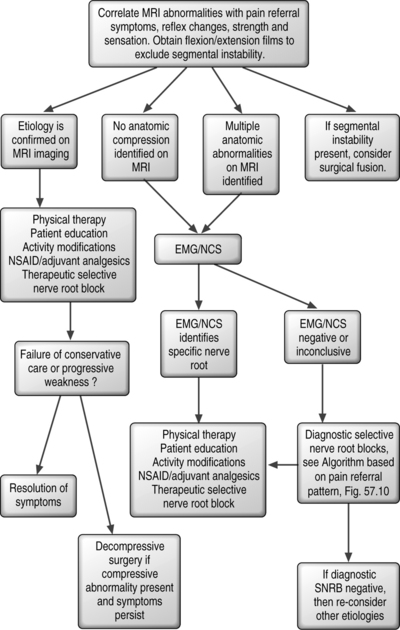
In patients with traumatic cervical radicular pain, multiple spinal and extraspinal structures may be injured. Degenerative spondylotic changes that are present on imaging often predate the new injury and sometimes mislead the clinician as to the pain generator. In some cases, a new herniated disc may make obvious the pain generator, but this is the case in only a minority of patients. Spinal structures such as the muscles, tendons and ligaments of the spine, cervical zygapophyseal joints, spinal nerve roots, and the intervertebral disc itself can become injured and cause axial neck pain with or without an extremity component which may mimic radicular pain. Extraspinal structures such as the shoulder, elbow, and wrist can become concomitantly injured and mimic or be present along with radicular pain. The diagnostic algorithm in this instance is more complicated and may require addressing multiple diagnoses (Fig. 57.9). This concept cannot be overemphasized. When making a medical diagnosis the law of parsimony is applied, but this rule could lead the spine clinician astray when evaluating a patient who sustained trauma. In determining the symptomatic spinal nerve root in patients with traumatic cervical radicular pain, attention to underlying degenerative narrowing may provide clues as to the spinal nerve roots that may be more readily injured. The patient’s pain referral pattern should be compared to known dermatomal and dynatomal maps, and examination should attempt to identify reflex changes and myotomal deficit to determine if the degenerative changes on imaging are of clinical relevance. In the absence of a correlation, electromyographic studies may be helpful to determine if radiculopathy is present. Unfortunately, in cases where reflexes and myotomal strength is normal, electromyographic studies are also often unrevealing. In these instances, fluoroscopically guided diagnostic selective nerve root anesthetization may be helpful in establishing a diagnosis of cervical radicular pain. Upon establishment of a diagnosis, therapeutic interventions such as pharmacologic measures, physical therapy, and therapeutic spinal injections can be implemented. If the affected nerve root has associated spondylotic changes that may be implicated as a compressive abnormality, surgical decompression can be considered in the event of failure of conservative care. However, in cases where post-traumatic radicular pain is present without evidence of an anatomic abnormality on MRI or CT-myelography, no specific surgical intervention is available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree