Chapter 53 Cerebral Palsy
The term cerebral palsy (CP) (originally “cerebral paresis”) was first used in 1843 by English orthopedic surgeon William Little in a series of lectures entitled “Deformities of the Human Frame.”110,207 As a result, CP was known for many years as “Little’s disease.” Other notable contributions include Sir William Osler’s The Cerebral Palsies of Children (1889), and publications by neurologists Sachs and Peterson (1890), and Sigmund Freud (1893). Although the term CP is well recognized in both the lay community and medical literature, a precise definition of what comprises CP is still debated to this day. In 2004 an international multidisciplinary group met to revise previous definitions of CP, and their contribution is as follows207:
Implicit in the above definition are several points of clarification that warrant further analysis. The notion of a permanent disorder excludes transient disorders of child development. Permanent does not equate to static, however, and the intent was to recognize that children and adults with CP can have changing patterns of clinical manifestations.207 The term disorder of development distinguishes CP from acquired brain injury that occurs after basic motor development has occurred. Central to the definition of CP is the presence of abnormal movement and posture. This concept has been universal in previous definitions. Because CP comprises a heterogeneous group of disorders, multiple etiologic mechanisms can result in the final common pathway of disturbance of development in the developing fetal or infant brain. There is no explicit upper age limit specified for the onset of the disturbance of brain development. The first 2 or 3 years of life, however, are identified as the crucial period for insults resulting in CP.207 Another key point in the definition of CP has been the identification of the brain insult to be nonprogressive. The pathophysiologic mechanisms leading to CP are presumed to arise from a single inciting event or a discrete series of events that are no longer active at the time of diagnosis.207 The disruption of the orderly process of early brain development in CP initiates a cascade of physiologic consequences potentially affecting every organ system in the developing child. The issues that arise are lifelong, dynamic in nature and require an interdisciplinary team and a “medical home” to effectively manage.13,25
Epidemiology
The most frequently reported incidence of CP is between 1 and 2.3 per 1000 live births.146 The above incidence often excludes acquired CP, which represents between 5% and 60% of all cases, the proportion correlating inversely with the degree of development of the country.91 Although early diagnosis is helpful clinically to initiate services, it can falsely elevate the incidence of CP, because up to 50% of children with CP diagnosed before 2 years of age can have spontaneous resolution of symptoms.56,144 Surveying population-based registers is also complicated by the use of alternate terms such as neonatal encephalopathy, birth asphyxia, periventricular leukomalacia (PVL), hypoxic brain injury, stroke, traumatic brain injury, and shaken infant syndrome.25
The ability to accurately identify trends in CP is dependent on consistent application of diagnostic criteria, and the availability of sufficient longitudinal data. In developed countries where sufficient data exist, the general trend has indicated a relatively stable incidence over a 30-year period from the 1960s to the 1990s. The trend has been a slow decline in CP from the 1960s to the lowest incidence in the 1970s, just before the widespread use of assisted ventilation. The ability to keep premature low birth weight (<2500 g) to extremely low birth weight (<1000 g) infants viable who might otherwise not have survived without mechanical ventilation, resulted in an incidence spike in the 1970s that had not returned to its previous low by the late 1990s.22 In addition, of children with CP diagnosed in the 1980s and 1990s, a greater proportion were more severely impaired.
Etiology
Injury to the immature brain resulting in CP can occur in the prenatal, perinatal, or postnatal period in both term and preterm infants. It is now thought that most cases of CP stem from injury incurred in the prenatal period.146 Common prenatal causes include TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex, other) or other infections, intrauterine stroke (ischemic and hemorrhagic), toxemia, and genetic malformation. The perinatal etiologies of CP comprise injuries occurring at or near the time of birth and can include placental abruption, cord prolapse, uterine rupture or similar processes that result in birth asphyxia. Fortunately, these etiologies are relatively rare, and multiple, controlled population-based studies have shown that an interruption of oxygen supply to the fetus does not account for most cases of CP.51,143,145,146,218 There are numerous postnatal etiologies of CP, including central nervous system (CNS) infection, cerebrovascular insults (ischemic and hemorrhagic), acquired head injury, anoxia, kernicterus, and progressive hydrocephalus. The etiology or pathophysiology of the brain lesion differs with gestational age and will determine the resultant CP subtype and associated movement disorder.
Premature birth is a well-recognized risk factor for CP, which results in an increased risk per infant up to 100-fold. The overall incidence of preterm and very preterm birth is quite low, however, and prematurity is implicated in half or less of CP cases.15,143 The most common CP subtype in premature infants is spastic diplegia. Although prematurity is a risk factor, the etiology of the CP is often multifactorial and a direct result of one or multiple environmental, genetic, or maternal factors that caused the preterm delivery (Box 53-1).
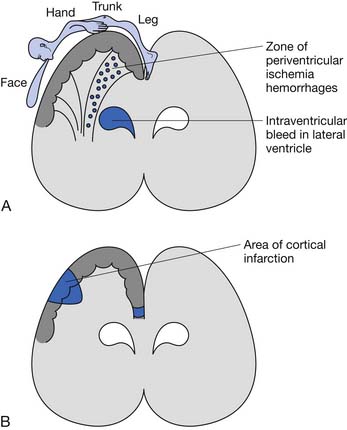
The underlying brain lesion in premature infants classically manifests as a white matter abnormality on neuroimaging. These white matter changes are a consequence of both arterial hemorrhage from fragile capillaries in the watershed zone next to the lateral ventricles known as the germinal matrix, and periventricular hemorrhagic infarction of venous origin. The highest risk for periventricular hemorrhage is between 23 and 32 weeks’ gestation.167 The extent of the bleeding into the germinal matrix is described by a grading system (Table 53-1). Intraventricular hemorrhage can be detected early in the clinical course via transfontanelle cerebral high-resolution ultrasound (US).44 Hemorrhagic infarction of venous origin in the periventricular region is usually asymmetric and located lateral to the external angle of the lateral ventricle.69,155 With healing of the periventricular hemorrhage, symmetric necrosis of the white matter adjacent to the lateral ventricles ensues, resulting in PVL (Figure 53-1). PVL is most commonly associated with a history of prematurity.16,102
Table 53-1 Grades of Intraventricular Hemorrhage in the Premature Brain
| Grade | Hemorrhage |
|---|---|
| 1 | Isolated to germinal matrix |
| 2 | With normal ventricular size |
| 3 | With ventricular dilatation |
| 4 | With parenchymal hemorrhage |
In term and near-term infants, hemiparetic and quadriparetic CP are the most common clinical subtypes of CP.143 Hemiplegic CP is frequently the result of a focal, or sometimes multifocal, lesion, most frequently caused by perinatal stroke or congenital malformation.16,219 The arm is usually more affected than the leg in hemiplegic CP, and seizures and cognitive issues caused by cortical injury can also be present. This is in contrast to PVL and diplegia, which is caused by a subcortical injury, and consequently is less likely to have associated seizures and significant cognitive sequelae.
Quadriplegic CP can be caused by any etiology that induces a diffuse bilateral insult to the brain, such as severe anoxic or ischemic brain injury. Intrapartum or birth asphyxia must be severe and prolonged to cause CP and accounts for less than 10% of quadriplegic CP cases.70,143 Because of the diffuse nature of injury to cortical and subcortical areas of the brain in quadriplegic CP, cognitive deficits, seizures, and the degree of disability are most severe in this group.
In addition to the brain lesions listed above, injury to particular areas of the brain can result in characteristic movement disorders such as those seen with basal ganglia lesions and athetoid CP. Athetoid CP is most often caused by hyperbilirubinemia and has seen a sharp decline in incidence with the institution of Rh incompatibility testing and treatment, and is now relatively rare.65 Basal ganglia and thalamic damage can also be associated with dystonia and associated dystonic subtypes of CP.16
Classification
The most appropriate way to classify children with CP continues to be debated. As early as 1862, Little111 described hemiplegic rigid, paraplegic, and generalized rigid types. In the 1950s, Perlstein156 and Minear134 discussed the importance of classifying children in a more comprehensive manner to include clinical symptoms, motor impairment, function, etiology, topography, degree of muscle tone, neuroanatomy, and therapeutic requirements. In 2000 the group for the Surveillance of Cerebral Palsy in Europe (SCPE; a network of CP surveys and registers formed in 14 centers in 8 countries across Europe) published its process of standardizing classification and descriptions of children with CP.204 Detailed neurologic and topographic categories include unilateral spastic CP, bilateral spastic CP, dystonic CP, choreoathetoid CP, ataxic CP, and nonclassifiable. This classification system is often used in the European literature, but further validity and reliability testing is needed.58
In the most commonly used classification system, tone patterns are described as spastic, dyskinetic, hypotonic, or mixed (Box 53-2), and distribution of limb involvement is characterized as diplegia (lower limbs affected more than upper limbs), hemiplegia (upper limb frequently more affected than lower limb), and quadriplegia (Figures 53-2 through 53-4). Triplegia has been used to describe a combination of diplegia and quadriplegia but with asymmetric upper limb involvement. As the upper limb involvement becomes more severe, inconsistencies in classifying the child as diplegic or quadriplegic arise. In addition, this classification system does not provide an adequate picture of the child’s functional or cognitive status.
The most widely used functional classification system is the Gross Motor Function Classification System (GMFCS), a five-level scale with four age bands that stratifies children based on gross motor ability (Box 53-3).150 This system has been shown to have excellent interrater reliability for both children younger than 2 years and children 2 to 12 years of age. The GMFCS does not describe the limbs affected or the type of movement disorder; consequently it is often used in combination with other descriptive classification systems. The GMFSC has been internationally recognized and is used frequently in research and clinical practice. Palisano et al.152 recently developed the expanded and revised GMFCS, which includes a 12- to 18-year age band and revision of the 6- to 12-year age band. Further validation and reliability testing is needed. An analogous scale to classify functional upper extremity use is the Manual Ability Classification System (MACS), a five-level scale that classifies how children with CP, ages 4 to 18 years, use their hands when handling objects in daily activities.49 It reflects the child’s typical manual performance and has been shown to have good validity and reliability. Its reliability in children younger than 5 years has recently been examined.160
BOX 53-3 Gross Motor Functional Classification System
Modified from Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39:214-223, 1997, with permission.
In 2004 an international multidisciplinary group met to critically review and revise the current classification system. A report on the “Definition and Classification of Cerebral Palsy April 2006” was subsequently published.169 It was recommended that four major dimensions of classification be used:
Diagnosis
The diagnosis of CP is based on the history and physical examination. Further diagnostic testing can help identify the most likely etiology, as well as potential comorbidities such as seizures. The diagnosis of CP is often made by 2 years of age12; however, children with the dystonic type or even mild hemiplegia might have more subtle findings that are overlooked until difficulty with more advanced skills becomes obvious.
History
Eliciting a thorough birth history (prenatal, perinatal, and postnatal) and developmental history is important in guiding one’s understanding of the etiology, determining underlying medical problems, determining the level of function, and developing a medical treatment plan. Delayed acquisition of motor milestones is a universal finding in children with CP. A delay in sitting is often a paramount concern of caregivers, and motor delay tends to increase with successive milestones.136 Early achievement of milestones such as rolling can be interpreted as precocious by caregivers but can be pathologic. A history of asymmetry in movement needs to be investigated. A history of regression in developmental milestones raises concern for progressive neurologic disease rather than CP, which is a static disorder. Other important aspects of the history include nutritional status, associated medical problems, visual and hearing status, therapies, equipment, education, and recreation. Obtaining a family history is essential. For example, children with slowly progressive familial spastic paraparesis can be clinically mistaken for having spastic diplegia, but an accurate family history can help differentiate the two.
Clinical Findings
The diagnosis of CP requires that a motor abnormality be present. Abnormalities in tone, reflexes, posture, and motor performance are seen in infancy.50 Other early predictive signs include weak cry and suck, irritability, and lethargy, although these are nonspecific. In infants born prematurely or of low birth weight, early neurologic abnormalities might not persist, such as transient dystonia.43,209 Spontaneous resolution of symptoms can occur in up to 50% of infants with CP diagnosed before 2 years of age.56,144 Because these early examinations might not predict future abnormality, repeated examinations and follow-up are necessary to ensure a correct diagnosis.
Persistent or obligatory primitive reflexes are an early sign of CP. Obligatory primitive reflexes are those that children cannot emerge from. These can often impede volitional motor control. Examples are the tonic neck reflexes including the asymmetric tonic neck reflex (Figure 53-5), symmetrical tonic neck reflex, and tonic labyrinthine reflex. The Moro (Figure 53-6), palmar, and plantar reflexes often persist in children with CP.138 An absent or inadequate Moro response on one side is found in infants with hemiplegia, brachial plexus palsy, or a fractured clavicle.
Abnormal tone can be manifested as decreased, increased, or fluctuating resistance to passive motion. Hypotonia is often a precursor to spasticity or athetosis.136 However, a prolonged period of hypotonia warrants further evaluation for a neuromuscular disorder such as congenital myopathy or spinal muscular atrophy. Only a small percentage of children are considered to have hypotonic CP.
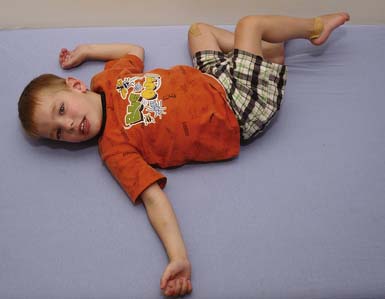
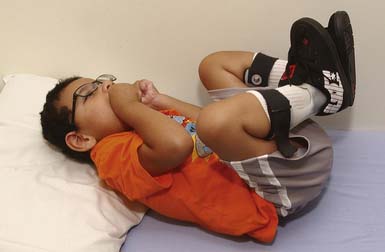
The spastic subtype of CP is the most common, affecting approximately 75% of children with CP. Spasticity is an increased resistance to passive motion that is velocity dependent.178 Upper motor neuron signs in these children include increased reflexes, clonus, and Babinski reflexes. Other findings include decreased strength and selective motor control with resultant impairments of coordination and balance. Common postural abnormalities are influenced by spasticity as well as primitive reflexes, and include lower limb extension and upper limb flexion patterns. The extension pattern of hip adduction, knee extension, and ankle plantar flexion can make diapering and dressing difficult. This pattern, also known as scissoring, is more prominent in weight-bearing or vertical suspension (Figure 53-7). Children with severe extensor tone can exhibit opisthotonic posturing with hyperextension of the neck and trunk (Figure 53-8). The upper limb flexor pattern consists of shoulder abduction, elbow flexion, wrist flexion, finger flexion, wrist pronation, and thumb adduction (Figure 53-9). This pattern becomes more pronounced when the child is stimulated or engaged in an activity resulting in a “high-guard” position. Abnormal fisting of the hand can be the only manifestation of increased tone in mildly affected children.
Fluctuating tone is more typical of dystonia, a movement disorder in which involuntary sustained or intermittent muscle contractions cause twisting and repetitive movements, abnormal postures, or both.178 It is often seen during rest. Spasticity and dystonia frequently coexist in CP, and differentiating the two movement patterns using clinical assessment is important to guide appropriate treatment. Children with dystonia have a greater degree of co-contraction, an increased resistance to external motion at slow velocities, and a greater impairment in muscle strength.108 When dystonia is present in infancy, one must have a high index of suspicion for treatable conditions such as glutaric aciduria type 1. Other dyskinesias include athetosis, an involuntary constant rotary or writhing movement pattern of the distal extremities (see Figure 53-9). Ataxia is rare, and if seen, should warrant further investigation into a degenerative process of the cerebellum. Progressive spinocerebellar diseases often present with a combination of ataxia and spasticity, and include Friedreich ataxia and ataxia telangiectasia
Patterns of movement can differ in children with CP. A combat crawling pattern is often used in children with CP because lower limb extensor tone makes reciprocal crawling difficult. However, this pattern is often used in “normally” developing children. Bunny hopping or knee walking is a pattern adopted for similar reasons. Toe walking is common in children with spasticity affecting bilateral lower limbs. A history of hand preference before 1 year of age, as opposed to normal handedness by the age of 2 years, or any asymmetry in movement should raise suspicion for a hemiplegia.136 Some children with hemiplegia scoot on their buttocks and propel themselves with their unaffected upper limb. Oral-motor abnormalities including drooling and tongue thrusting can be present.
Diagnostic Studies
Neuroimaging
Neuroimaging is part of the diagnostic evaluation of CP and can provide insight into the etiology, medical management, and neurodevelopmental outcome. For example, brain malformations found on magnetic resonance imaging (MRI) in children with spastic CP have been associated with epilepsy and mental retardation,103 and can warrant further investigation into a genetic etiology. Children with a congenital hemiparesis and a finding of a cerebrovascular infarct on neuroimaging might prompt further investigation and identification of a treatable coagulopathy.
The most commonly used modality in premature infants is cranial US for the evaluation of PVL. Guidelines for cranial US have been published by the American Academy of Neurology (AAN), and screening is recommended in premature infants less than 30 weeks’ gestation at 7 to 14 days of age and again at 36 to 40 weeks. US is used to counsel families about prognosis and has been correlated with neurodevelopmental impairment.8,215 However, the positive predictive value for CP with US in one study was only 48%.44 Diffuse, noncystic white matter abnormalities often seen in preterm infants are poorly visualized on US and are better seen on MRI.86
The AAN has recommended that routine neuroimaging, preferably with MRI over computed tomography, be performed in children with suspected CP if an etiology has not been determined.12 MRI has been shown to have a high yield of greater than 80% in identifying abnormalities in children with CP,12,167 and can aid in determining whether the injury was prenatal, perinatal, or postnatal in onset.222 Significant associations have been found between cerebral white matter and gray matter abnormalities on MRI and neurodevelopmental impairment at 2 years of age in preterm infants less than 30 weeks’ gestation.216 Significant correlations between MRI findings and GMFCS in children with diplegic and quadriplegic CP have been described.104 Wu et al.219 found that children with congenital hemiparesis and normal cranial imaging studies were more likely to outgrow clinical signs of hemiparesis by 3 years of age.
Coagulation Studies
Children with hemiplegic CP have a high incidence of prenatal and perinatal cerebral infarction. Studies show that cerebrovascular occlusion, often in the middle cerebral artery, occurs in up to 37% of children.139 Coagulation abnormalities are found frequently in these children and include factor V Leiden mutation, protein C or S deficiency, elevated lipoprotein-a levels, the presence of anticardiolipin or antiphospholipid antibodies, and G20210A mutation of prothrombin.68,185 The AAN recommends that diagnostic testing for a coagulation disorder be considered, and this has been supported in other studies.12
Functional Prognosis
Although CP is a nonprogressive disease, the differences in clinical course are considerable and characterized by a wide change in function over the years of growth.41 Motor milestones are not always met at a predictable rate, and caregivers frequently have questions regarding ultimate functional prognosis. Attempting to predict function becomes important when identifying future care, and therapeutic and equipment needs.
The attainment of independent sitting by 2 years of age is a good predictive sign for eventual ambulation, whereas children who are unable to sit by 4 years of age rarely ambulate.135 In addition, the presence of fewer than three primitive reflexes at 18 months of age is a good prognostic indicator for ambulation.138 Gross motor development has been shown to typically reach a plateau at 7 years of age.17,170 Molnar and Gordon138 reported that walking is achieved in 80% to 90% of children with diplegia, 50% of those with quadriplegia, and 75% of those with dyskinesia. In 2008, walking ability and predictive factors were described in a group of 9012 children with CP from the SCPE common database.17 By 5 years of age, walking with or without aides was seen in 96% of children with unilateral spasticity, 57% of the bilateral spastic group, 90% of the ataxic group, and 61% of the dyskinetic group. It is difficult to compare the statistics in the latter study to those reported by Molnar and Gordon because of the differences in CP classification and nomenclature; however, walking ability was significantly related to cognitive status in both studies. Independence in activities of daily living is often achieved in children with diplegia and those with quadriplegia who are ambulatory.136
Several studies have examined the stability of the GMFCS over time. It has been shown that 73% of children aged 16 months to 13 years remain in the same GMFSC level.151 Children in levels I and V are least likely to be reclassified, and children younger than 6 years tend to be reclassified to a lower level. The predictive value of the GMFCS enables health professionals to give families a more reliable functional prognosis. The GMFCS classification of infants is more challenging, however, because of their variability in development, making the prognostic value less in this group. It has been found that 42% of children younger than 2 years had a change of one or two GMFCS levels by 2 to 4 years of age.
As children reach adulthood, walking can become less efficient because of physiologic changes such as development of contractures, joint degeneration, or change in body weight. The energy cost of walking has been found to correlate with the GMFCS level.92 Fifty-two percent of adults, aged 24 to 76 years, with hemiplegic and diplegic CP report deterioration in walking function.149 Deterioration was associated with increased pain frequency and intensity, reduced balance, and fatigue. Stability of the GMFCS in adults with CP, aged 17 to 38 years, has also been examined and shows that the GMFCS level at 12 years of age is highly predictive of adult motor function.123
Medical Management
Feeding, Growth, and Nutrition
Growth and nutrition disorders are very common in children with CP and are the result of a combination of factors. It has been reported that 27% of children with moderate to severe CP are malnourished,174 which leads to slower growth than expected compared with age- and gender-based standards.175,176,198 Although the incidence of malnutrition is higher in persons with spastic quadriplegia and more severe disability, one study found that up to 30% of children with spastic hemiplegia and diplegia were undernourished.192 Malnutrition in CP is known to have a negative impact on the physiology of nearly every organ system and has been demonstrated to increase health care utilization, decrease societal participation, impair motor function, decrease life expectancy, negatively affect bone health, impair wound healing, and increase the risk for postoperative complications.105 The importance of optimizing nutritional health cannot be emphasized enough.
Poor oral-motor function results in dysphagia, malnutrition, and dehydration, and increases the risk for aspiration pneumonia. At least 75% of children with CP have been reported to have some sign or symptom of dysphagia.164,165,193 Gastrostomy tube (G-tube) placement is one means to ensure adequate nutrition intake and decrease the risk for aspiration, and is a routine practice in the medical management of CP. Despite proven efficacy, G-tubes are generally used only after other treatments have failed.26,39 Clinicians must be sensitive to some caretaker’s perception of G-tube feeds as unnatural.157 Despite initial reluctance, most caretakers are usually pleased with the outcome after surgery given the improvement in the child’s health.203
Although nutritional concerns have been demonstrated to be a significant contributor to poor growth in CP, they are certainly not the only factor. Hormone assays in children with CP have identified growth hormone deficiency.38 In addition, children with moderate to severe CP have been found to lack the dramatic pubertal growth spurt that accounts for a significant amount of adult height. Children with CP often experience early adrenarche that lasts longer than their peers.217 Additional work has suggested that pubertal girls with CP, even when adjusting for nutritional status, have decreased insulin-like growth factor-1 and growth hormone levels.105 The etiology and clinical significance of endocrine abnormalities in CP warrants further research.
Pulmonary
The leading cause of death in children with a severe disability, including CP, is chronic respiratory infection, and more than 90% of deaths in children with severe CP are caused by pneumonia.79,161 The etiology of pulmonary compromise in CP is multifactorial, including pulmonary aspiration, decreased mucociliary clearance, suppuration, kyphoscoliosis, and airway obstruction.54 Various tests are available to evaluate for aspiration, including the salivagram, barium videofluoroscopy, fiberoptic endoscopic evaluation of swallowing, and a radionuclide scan. If aspiration of saliva and drooling is deemed problematic, management can include oral-motor therapy, behavior modification via biofeedback, anticholinergic medications such as glycopyrrolate, benztropine, and scopolamine transdermal patches, botulinum toxin injections to the salivary glands, surgical resection of the salivary glands, or rerouting of the salivary ducts.23,130 Treatments to improve clearance of secretions can include inhalation therapy with normal or hypertonic saline, bronchodilators, and chest physiotherapy.
Pulmonary infections are common in CP, and a low threshold should be had for antibiotic use when there is evidence of suppurative lung disease. The antibiotic selection should be in response to sputum culture results and will often require a prolonged course of treatment (3 to 4 weeks).54 Immunization against influenza and pneumococcus is also recommended if not otherwise contraindicated. Kyphoscoliosis in CP can also contribute to pulmonary dysfunction usually as a result of structural restrictive lung disease. Airway obstruction is also a frequent contributor to respiratory dysfunction and can involve either the upper or lower airways. Upper airway obstruction can occur at the supraglottic level, with redundant aryepiglottic folds causing a clinical picture of laryngomalacia. Lower airway obstruction might be the result of reactive airway disease or asthma, or the consequence of intrinsic lung abnormalities. A trial of bronchodilators and asthma therapy is warranted if reactive airway dysfunction is thought to contribute, but should be monitored for efficacy and clinical response.
Respiratory dysfunction in CP might also affect sleep, and community-based surveys have identified a higher prevalence of sleep breathing problems in children with CP compared with healthy children.99 Treatment of the sleep disturbance involves directed therapy to address the identified contributing factors. The disruption of normal sleep patterns has an enormous effect not only on the cognition and quality of life of the child, but also on the caretaker, parents, and siblings who are involved in the provision of care.54
Neurologic Issues
The disruption of the normal orderly sequence of early brain development in CP is associated with a multitude of neurologic comorbidities. A practice parameter guideline by the AAN in 2004 recommended screening children with CP for associated mental retardation, ophthalmologic and hearing impairments, speech and language disorders, and oral-motor dysfunction.55 Children with CP also have an increased frequency of hydrocephalus, sleep disturbance, and epilepsy.
On average, 43% of children with CP develop epilepsy, and the risk is increased in those with neuroimaging abnormalities. The reported incidence of epilepsy is variable, with an incidence of 50% to 94% in spastic quadriplegia, 30% in hemiplegia, and 16% to 27% in diplegia or ataxic CP.12 Despite this, there is no evidence to support routine electroencephalogram (EEG) screening in children without a history of seizures, and EEG was not found to be helpful in identifying the etiology of CP.12
Mental retardation or the impairment of cognitive and neuropsychological function is commonly present in CP.53 In general terms the degree of motor impairment and the extent of cortical injury on imaging studies directly correlate with the severity of cognitive deficits, with the exception of dyskinetic CP where this association is lacking.53 There is also a strong correlation of greater intellectual impairment in children who have abnormal EEG findings or epilepsy.83,213 A comprehensive neurodevelopmental evaluation including speech-language assessment and neuropsychologic evaluation might help in guiding therapeutic intervention and tailoring the school program.
Visual impairment in CP is common (28%), and the relative risk is increased with the degree of imaging abnormality.12 The etiology is often multifactorial, including pathologic disorder. of the anterior afferent sensory visual pathways, ocular structures, cortical vision impairment (CVI), and disorders of ocular motility. CVI is commonly defined as a loss in visual function in the absence of damage to the anterior afferent visual pathways or ocular structures.95 The term CVI is not accurate because the visual impairment is not limited to the visual cortex but also usually involves white matter pathways such as the optic radiations in children with PVL.82 Neuroimaging findings have been found to correlate with the prognosis, as children with isolated striate cortex damage had a better prognosis than did those with isolated PVL.95 The most common etiologies for CVI are perinatal hypoxia (35%), prematurity (29%), hydrocephalus (19%), structural CNS abnormalities (11%), and seizures (10%).95 With the increased survival of premature and very sick term infants as a result of advances in modern perinatal care, the prevalence of CVI in childhood has been steadily rising over the past few decades. CVI is now the most common cause of acquired bilateral visual impairment in childhood in developed countries.55,195 The overall prognosis for significant improvement in visual function with CVI is poor, and children with structural abnormalities of the brain and afferent visual pathways are the least likely to improve.95
Hearing impairment is reported to occur in approximately 12% of children with CP.95 Risk factors include very low birth weight, kernicterus, neonatal meningitis, severe hypoxic-ischemic insults, and administration of ototoxic medications. The 2008 U.S. Preventive Services Task Force recommends universal screening for hearing loss in all newborn infants.211
Speech and language disorders are common in CP, with a reported incidence of articulation disorders and impaired speech intelligibility of approximately 38%.36,113 Anarthric or dysarthric speech is caused by oral-motor dysfunction as a result of bilateral corticobulbar dysfunction or lesions to cortical speech-language centers. Many children with CP have preserved receptive language despite being nonverbal or markedly dysarthric. Marked motor and speech impairment with relative preservation of intellect is the hallmark of athetoid CP secondary to subcortical basal ganglia lesions. This is important to remember because one should assume that children understand what is said to them or in their presence. In children with significant expressive language dysfunction, alternative means of communication including yes-no sign boards, picture boards, adaptive switches, or augmentative communication devices should be explored.
Genitourinary
Voiding dysfunction has been estimated to affect 36% of children with CP.129 Symptoms can include urinary frequency, incontinence, or difficulty urinating. In studies where urodynamic evaluation was performed, neurogenic detrusor overactivity with a low bladder capacity was seen in 47% of children with CP, while the finding of detrusor-sphincter dyssynergia was present in 11%.93 In symptomatic children, early referral for urodynamic evaluation and treatment might significantly improve urinary symptoms.
Gastrointestinal
Gastrointestinal symptoms are frequent in CP and are often attributable to impairments in colonic motility.153 Gastroesophageal reflux can be seen because of weakness of the lower esophageal sphincter and can result in emesis and aspiration. Malnutrition as a consequence of oral-motor dysfunction can further complicate the impaired colonic motility that results from inadequate fluid and dietary fiber intake. The consequence of impaired colonic motility is chronic constipation with possible long-term large bowel megacolon and volvulus, which are preventable with regular bowel evacuation. Abnormal segmental colonic transit times have been correlated with ambulatory function in CP.153 Fecal incontinence or defecation distress can occur because of anal sphincter or pelvic muscle incoordination. This dysfunction in the pelvic floor musculature is thought to be the result of abnormal neuromotor control and can be assessed with anorectal manometry.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree