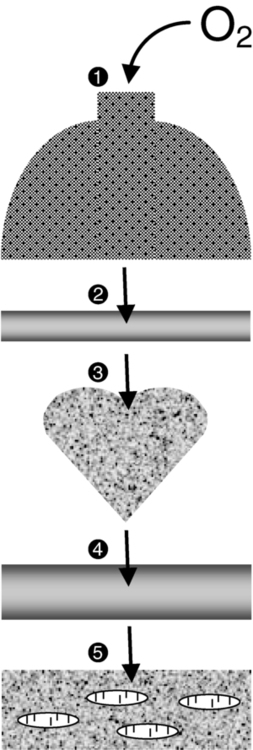
After reading this chapter, the student or therapist will be able to: 1. Explain the physiological principles related to cardiovascular responses to exercise testing. 2. Discuss the evidence behind cardiovascular fitness and describe the factors that contribute to the deconditioned state in adults with neurological disorders. 3. Explain the adaptive responses to aerobic training in populations with neurological disorders and the factors underlying these responses. 4. Discuss general guidelines for designing exercise programs to improve cardiovascular health and fitness. The cardiopulmonary health of individuals with residual movement dysfunction after a neurological insult is now regarded as a topic of interest in neurorehabilitation. In traditional practice, the state of the neuromuscular system preoccupied the attention of clinicians in the quest to optimize neurological recovery. Most interventions were based on strategies to improve the capacity of that system—an approach that has met with limited success in terms of restoring functional independence. It is now clear that recovery cannot be explained solely on the basis of improved neuromuscular function. For example, Roth and colleagues1 determined that less than one third of the variance in functional limitations after a stroke can be explained by the extent of neurological impairment. Nevertheless, the current approach to neurorehabilitation is somewhat puzzling. Evidence has accumulated indicating that many people with neurological disabilities are woefully deconditioned. There has been widespread acknowledgement of the central role that aerobic exercise plays in improving cardiopulmonary health and fitness. Furthermore, application of the principles of exercise physiology in cardiac rehabilitation has been widely endorsed. Yet neurorehabilitation clinicians have been observed to practice without full knowledge of their patients’ cardiac status or without monitoring heart rate (HR) and blood pressure (BP).2 Moreover, there is evidence to suggest that patients with neurological insults have not been challenged enough in therapy to induce the metabolic stress needed to enhance their cardiopulmonary fitness.3,4 A troubling explanation offered for these observations is that clinicians lack either an understanding or an appreciation of the basic physiological principles of exercise.5,6 This chapter begins with an overview of physiological principles related to cardiovascular responses to exercise testing. A summary of the evidence of cardiopulmonary fitness levels in adults and children with neurological disabilities is followed by a description of factors that contribute to the deconditioned state. Possible mechanisms responsible for reduced exercise capacity are then reviewed. Adaptive responses to aerobic training in patients with neurological conditions are examined, as are factors underlying these responses. The chapter closes with a summary of guidelines for the design of exercise programs that can be used to improve cardiopulmonary health and fitness. Appendix 30-A at the end of this chapter clearly identifies the meanings of the abbreviations used throughout the chapter. At rest the human body consumes roughly 3.5 mL of oxygen (O2) per kilogram per minute, or 1 metabolic equivalent (MET).7 In the resting state, skeletal muscle activity accounts for less than 20% of the body’s total energy expenditure; the brain, making up only 2% of body weight, also consumes 20% of the available O2.8 Activities at rest such as breathing and contracting of the heart can be sustained indefinitely because the power demands of these activities are met by the rate of energy turnover. In other words, these activities occur well below the critical power of the muscles, defined as the maximal rate of work that can be endured indefinitely.9 Any physical activity beyond the resting state requires more O2; the increase is dependent on the intensity of the effort involved. The rise in metabolism relies on O2 transport by the pulmonary and circulatory systems and O2 usage by the active skeletal, cardiac, and respiratory muscles to convert chemical potential energy to mechanical energy.10 The components of the O2 transport system are outlined in Figure 30-1. Selective distribution of the increased blood flow to regions with heightened metabolic demands—the working muscles—is largely a result of local vasodilation mediated mainly by metabolites acting on the vascular smooth muscle (e.g., carbon dioxide [CO2], hydrogen ions [H+], nitric oxide, potassium ions, adenosine) and vasoconstriction in tissues with low metabolic demands.11 Blood flow to other vascular beds (e.g., renal and splanchnic bed) either is unchanged or decreases through active vasoconstriction resulting primarily from increased sympathetic discharge. Cerebral autoregulation maintains regional and total cerebral blood flow and normal tissue oxygenation over a wide range of BPs12; thus, cerebral blood flow and O2 delivery during exercise either remain stable13,14 or increase slightly.15,16 As exercise intensity increases, systolic BP (SBP) increases markedly, whereas diastolic BP (DBP) either remains unchanged or lowers slightly, resulting in a moderate increase in mean arterial pressure.17 Extraction of O2 from the muscle capillary blood to mitochondria is dependent on an adequate O2 diffusion gradient. During a progressive increase in workload, the arterial hemoglobin saturation and arterial O2 content remain relatively constant, whereas the venous O2 content decreases substantially as a result of increased O2 extraction in the active muscles.18 As the metabolic rate rises, the minute ventilation (i.e., respiratory rate multiplied by the tidal volume) increases to remove CO2 and to regulate pH balance of the active muscles. At low-intensity exercise, ventilation (mainly tidal volume) increases in a linear manner relative to the volume of O2 use (Vo2) and CO2 production (Vco2). Above the critical power, the energy demand of the muscle exceeds the capacity of the aerobic process to supply energy for muscle contraction; the additional energy is supplied by the anaerobic glycolytic system. During more intense exercise, ventilation is extremely variable among individuals; the respiratory rate usually increases without a substantial change in tidal volume.17 The point at which the rate of glycolysis exceeds that of oxidative phosphorylation is called the anaerobic threshold (which approximates the ventilatory threshold or lactate threshold).17 Pyruvic acid is converted to lactic acid, which completely dissociates to lactate and H+, resulting in a rise in blood lactate levels and a fall in intramuscular pH. Exercise-induced muscular fatigue is caused by the exponential accumulation of lactate and a drop in intramuscular pH, with negative effects on the actin-myosin turnover rate, enzyme activities, and excitation-contraction coupling. Maximal oxygen consumption (Vo2max) is defined as the highest O2 intake an individual can attain during physical work.17 The Fick equation describes the relationship between cardiovascular function and Vo2max: Thus Vo2max reflects both O2 transport to the tissues and O2 usage by the tissues. Increases in Vo2 during exercise are caused by increases in both cardiac output and a-vO2diff, with HR and stroke volume (SV) increasing progressively over the lower third of the workload range. Thereafter, HR continues to increase while SV remains essentially constant,19,20 resulting, at maximal effort, in a cardiac output three to six times greater than baseline levels. An increase in SV (50% over resting volume) is caused by enhanced myocardial contractility and increased venous return resulting from compression of the veins by contracting muscles and reduced intrathoracic pressure.21 At low-intensity exercise the increase in HR is mainly a result of decreased vagal tone, but as exercise intensifies, sympathetic stimulation and circulating catecholamines play a greater role, yielding, at maximal workloads, a rise in HR 200% to 300% above the resting level.22 Exercise (aerobic) capacity is the principal determinant of the ability to sustain the power requirements of repetitive physical activity. Vo2max is generally accepted as the definitive index of exercise capacity and cardiopulmonary fitness.23 Vo2max is a relatively stable measurement; variability of repeated measures of Vo2max has been reported to be 2% to 4%24 or 0.2 L/min.25 Accurate determination of Vo2max requires (1) adequate duration and work intensity by at least 50% of total muscle mass, (2) independence from motivation or skill of the subject, and (3) controlled environmental conditions.26 Also, because test performance is sensitive to time of day, the time of repeat testing should be consistent. Before any fitness test, a 3- to 5-minute warmup of slow treadmill walking on a level grade or unloaded pedaling that raises the metabolic rate twofold above resting should be performed.27 A proper warmup prevents excessive local muscle fatigue from occurring before Vo2max has been attained.28 Furthermore, a 3- to 5-minute cool-down should follow test completion to aid in venous return to prevent blood pooling in the peripheral vasculature and a subsequent drop in DBP. The intensity of exercise can be increased in a continuous progressive manner (i.e., step or ramp protocol) or, less commonly, in a discontinuous progressive manner (i.e., subject rests between stages). Throughout testing, continuous monitoring of the electrocardiogram and periodic monitoring of BP are essential. The optimal duration of a graded exercise test is 8 to 12 minutes, with testing terminated when the subject can no longer generate the required power, is limited by symptoms, or is unable to continue safely.29 Variables of interest during exercise testing include Vo2max expressed in absolute terms (liters of O2 per minute) or relative to body mass (milliliters of O2 per kilogram of body weight per minute), MET level, percent of predicted HRmax, respiratory exchange ratio (RER; ratio of Vo2 to CO2), peak power, minute ventilation, tidal volume, respiratory rate, and rating of perceived exertion (RPE) according to the Borg scale.30 Because there is considerable variability in HRmax among healthy individuals, the percent of predicted HRmax attained is not a robust indicator of exercise capacity.31 Similarly, because both total exercise time and peak exercise intensity (or power attained, i.e., peak treadmill speed and grade or peak power on bike) are dependent on the test protocol, neither is a reliable measure of exercise capacity.32,33 In addition, noninvasive estimation of the anaerobic threshold by identifying the point of nonlinear increases in minute ventilation and Vco2 can be highly subjective and thus unreliable.34 The principal marker of exercise capacity is attainment of a plateau in Vo2 beyond which there is a change of less than 100 mL/min, with further increases in workload dependent solely on anaerobic metabolism.29 In cases in which a Vo2 plateau is not observed (typically in deconditioned or elderly individuals or in patients with heart disease), the preferred term for the value obtained is peak Vo2 (Vo2peak).35 Criteria for attainment of Vo2peak include achieving the age-predicted HRmax, RER in excess of 1.15, minute ventilation greater than the predicted maximal voluntary ventilation, tidal volume greater than 90% of the inspiratory capacity, and obvious patient exhaustion.28 The modality of testing (e.g., treadmill walking, cycling, stepping, arm cranking) can affect Vo2max values. The treadmill has the greatest potential to recruit sufficient muscle mass to elicit a maximal metabolic response, particularly in deconditioned individuals.26 Bike ergometry yields 85% to 90%, and arm ergometry 70%, of the Vo2max achieved with a treadmill.26 Ideally the mode of exercise should be consistent with the patient’s typical activity. Thus the treadmill is often preferred because the pattern of muscle activation during treadmill walking is similar to that for most mobility tasks. In patients with neuromuscular conditions, however, impaired balance and motor control often preclude the use of standard treadmill testing protocols. To resolve this limitation, we devised and validated an exercise protocol using a body-weight support system to permit safe and valid testing of Vo2max early after stroke.36 For subjects with paraplegia, tests with wheelchair treadmills are more functionally relevant than those using arm ergometry. Although submaximal tests do not measure the systemic response, they are inexpensive to administer and have a low risk of adverse events. The essentially linear relationship between Vo2 and HR permits the estimation of Vo2max from HR measurements taken during submaximal exercise. For example, for healthy people the HR increases approximately 50 beats per uptake of 1 L of O2, independent of sex and body size.37 For unfit individuals and patients with cardiac impairment, the increases in HR are greater per liter, except for patients taking β-blockers, who demonstrate blunting of the HR response throughout exercise. The Åstrand-Ryhming nomogram is often used to predict Vo2max from submaximal HR.38 The HR-Vo2 relationship is independent of the exercise protocol. However, HR, unlike Vo2max, is markedly affected by many stresses (e.g., dehydration, changes in body temperature, acute starvation), resulting in substantial error and inaccurate Vo2max estimations.26 In fact, discrepancies between estimated and measured Vo2max in individuals with low exercise capacity can be as high as 25%.39 Documentation of exercise capacity in populations with neurological disorders has been hindered by the lack of testing protocols that can safely and effectively accommodate the motor and balance disturbances common to these populations. Not surprisingly, the limited evidence to date suggests that most individuals with neurological disabilities are significantly deconditioned. A summary of Vo2peak data from studies of common neurological conditions is presented in Table 30-1. Variability in the results from a multitude of factors, including differences in testing protocols, as discussed in the previous section, and differences in subject characteristics; these points are discussed in the following section. TABLE 30-1 EXERCISE CAPACITY IN COMMON NEUROLOGICAL CONDITIONS People with high fitness levels use only a small fraction of the physiological fitness reserve40 of the cardiovascular, respiratory, and neuromuscular systems to respond to the metabolic challenge of activities of daily living (ADLs).41,42 Thus, small declines in exercise capacity may not be noticeable in carrying out daily activities. In contrast, relatively minor reductions in capacity can substantially influence performance of ADLs by deconditioned individuals. Light instrumental ADLs require approximately 10.5 mL of oxygen per kilogram per minute (3 METs), whereas more strenuous activities have metabolic costs of about 17.5 mL/kg/min (5 METs).43 Cress and Meyer44 reported that the Vo2peak of 20 mL/kg/min is needed for older adults to meet the physiological demands of independent living. From the data presented in Table 30-1, it is evident that many people living with neurological disabilities (particularly stroke, tetraplegia, and postpoliomyelitis syndrome) do not have the level of fitness required for the more strenuous ADLs and independent living. Moreover, relative exercise capacities (expressed as a percentage of normative values) associated with the disabilities in Table 30-1, with the exception of Parkinson disease, are of concern, given that Vo2peak values less than 84% of normal are considered pathological.45 For individuals with neurological disabilities, the minimum Vo2 requirements for ADLs are actually greater than the previously mentioned levels because of the increased energy requirements resulting from gross motor inefficiencies and other related factors.46–48 In other words, the percentage of Vo2peak required for activity at a fixed submaximal workload (termed fractional utilization) is increased. When the anaerobic threshold is exceeded prematurely and lactate accumulation is accelerated, accomplishment of low-intensity ADLs is unsustainable for extended periods and achievement of mid- to upper-intensity ADLs is virtually impossible. Moreover, the combination of poor exercise capacity and elevated energy demands results in diminished reserves to support other activities. For example, in the case of people with postpoliomyelitis syndrome, the energy costs of walking are about 40% higher than for healthy peers and are highly correlated with lower-extremity muscle strength.49 Thus in the calculation of fractional utilization for walking, the numerator (Vo2 during walking) is increased and the denominator (Vo2peak) is decreased; hence, fractional utilization is substantially increased. Of the neurological populations, people poststroke are the largest consumer group in need of rehabilitation services. This group also has received the most attention in the literature with regard to functional capacity. Exercise capacities documented in this population are consistently low—from 8.3 mL/kg/min in the subacute period50 to 22.4 mL/kg/min in the chronic period.51 As much as 75% to 88% of Vo2peak (almost twice that of the healthy control subjects) is required to perform household chores52 and one-and-a-half to three times the Vo2 levels of healthy controls are needed to walk on level ground.46,53,54 Not surprisingly, up to 70% of patients complain of fatigue after stroke55 and rate poor energy levels ahead of mobility limitations, pain, emotional reactions, sleep disturbances, and social isolation as the area of greatest personal concern.56 In addition to contributing to reduced ADL performance and increased fatigability, low fitness levels are associated with higher mortality. Exercise capacity has been reported to be an independent predictor of mortality in persons with coronary artery disease (CAD), a comorbidity prevalent in some populations of people with neurological conditions.57,58 Those with a Vo2peak <21 mL/kg/min are classified as the high-mortality group and with greater than 35 mL/kg/min as the excellent-survival group.59 Thus, determining an individual’s Vo2peak is of clinical value. Individuals who are being encouraged or are internally motivated to perform beyond their capacity and beyond the capabilities of the interaction of multiple systems are in a high-risk category. Conversely, individuals who are undermotivated or depressed and are performing below their capacity can be trained to self-monitor, which empowers them to reach goals that are safe and have the potential to improve the quality of their lives. To identify appropriate measures to improve fitness levels in people with neurological disorders, the myriad factors at play that contribute to the deconditioned state must be considered. A useful conceptual framework to discuss the interaction of these factors is the International Classification of Functioning, Disability and Health (ICF)60 (see Chapter 1). The ICF uses a biopsychosocial approach to organize factors related to the health conditions into two components: (1) personal and environmental contextual factors and (2) functioning and disability, which are further subdivided into components of body functions and structures, activity, and participation (Figure 30-2). Through application of the ICF framework, the complexity of interacting influences on cardiovascular and pulmonary health and fitness becomes more understandable. A decline in Vo2max of approximately 1% per year (0.4 to 0.5 mL/kg/min/year) occurs from 25 to 75 years of age.61 In accordance with the Fick equation, a reduction in Vo2max is caused by reductions in both O2 transporting (i.e., Qmax) and O2 utilization capacity (i.e., a-vO2diffmax) associated with cardiac, respiratory, and muscular changes. Decreased Qmax is the result of increasing myocardial stiffness and decreased left ventricular contractility, manifested by reductions in both ejection fraction and HRmax—hallmarks of cardiovascular aging.62 In fact, the reduction in HRmax, which decreases 6 to 10 beats per minute (bpm) per decade, is responsible for much of the age-associated decline in Qmax.63 Evidence also suggests that older adults have a smaller SVmax63 and that BP and systemic vascular resistance are higher during maximal exercise in older versus young adults.64 With advancing age, reduced elastic recoil of the lung and calcification and stiffening of the cartilaginous articulations of the ribs restrict compliance of the lungs, thus limiting increases in minute ventilation during exercise.65 Age-related decline in oxidative capacity of the working muscles and hence decreased a-vO2diff during peak exercise66 have been attributed to alterations in mitochondrial structure and distribution, oxidative enzyme activity,67 and skeletal muscle microcirculation, as well as sarcopenia resulting from a reduced number and size of fibers, particularly type II fibers.68 Nevertheless, despite loss of aerobic capacity with aging, people without chronic health conditions retain adequate reserves for daily activities. However, for aging individuals with a neurological impairment, the decrease in aerobic capacity with age can further reduce their reserves and thus threaten living an independent lifestyle. In fact, in a population-based study, age was found to be a significant independent predictor of recurrent stroke.69 Particularly disadvantaged are people with cerebral palsy (CP) or other developmental disabilities as a result of an incomplete development of their musculoskeletal and cardiorespiratory systems at the time of the neurological event, which accelerates the aging process.70 In the case of people with Down syndrome, however, Baynard and colleagues71 found that age-related changes in exercise capacity did not follow the typical pattern of decline after the age of 16 years. People with spinal cord injury (SCI) are at an increased risk for cardiac and respiratory complications with age.72 The absolute and relative Vo2max of women is about 77% of that of men, after adjustment for body weight and activity level.73 For nondisabled older adults, Kohrt and colleagues74 reported no significant gender difference in the percentage of improvement in Vo2max; women had an increase in Vo2max of 26% ± 12% (range 4% to 58%), and men demonstrated a 23% ± 12% (range 0% to 51%) increase. Although older men and women generally exhibit similar responses to maximal exercise, older women tend to have lower SBP during maximal exercise.64 Smoking is one factor that has been shown to impair exercise capacity in the general population.76 Smoking causes increases in HR, myocardial contractility, and myocardial oxygen demand, which can lead to atherosclerosis and acute cardiovascular events.77 In the stroke population, smoking doubles the risk of death (equivalent of a 7-year reduction in life span) when compared with the risk in nonsmokers and ex-smokers.78 Currently the relationship between cardiovascular disease and diet is receiving international attention.79 Indeed, obesity increases the risk of cardiovascular risk factors such as impaired glucose tolerance and type 2 diabetes, hypertension, and dyslipidemia.80 Specifically, abdominal obesity not only increases the risk of atherosclerotic disease but also the risk of primary ischemic stroke.81,82 In addition, when compared with corresponding normal-weight populations, overweight youth with SCI and spina bifida have lower cardiovascular fitness.83 Yet the lifestyle factor that has received the most attention in the literature is habitual activity. There is now irrefutable evidence of the link between physical activity and cardiopulmonary health and fitness.76,84–86 In fact, in the stroke population, prestroke physical activity has been found to decrease stroke severity as well as to result in better long-term rehabilitation outcomes.87 Cardiovascular alterations resulting from physical inactivity (i.e., reduced Vo2max and Qmax) parallel, in many ways, the changes that occur with aging; in fact, sedentary lifestyles explain a significant proportion of these age-related declines. If physical activity levels and body composition remain constant over time, the expected rate of loss in aerobic power associated with senescence is reduced by almost 50%.68 Nonetheless, people with chronic health conditions often rate poorly in terms of daily physical activity, in part because of underlying physical impairments (e.g., paralysis, pain). For example, people with SCI spend as little as 2% of their walking time participating in leisure physical activity,88 making them the most sedentary members of society.89 Some people with multiple sclerosis (MS) avoid physical activity to prevent elevated body temperature and minimize symptoms of fatigue.90 Inactivity can lead to increased cardiovascular risk factors such as hypertension and dyslipidemia as seen in youth with chronic disabilities (including CP and SCI).91 Bernhardt and colleagues92 found that after a stroke, patients spend more than 50% of their time resting in bed. Short periods of bed rest cause rapid decreases in aerobic capacity—a 15% reduction in healthy, middle-aged men after 10 days of recumbency93 and a 28% reduction in healthy young subjects after 3 weeks.94 Inactivity-induced reductions in Vo2peak have been attributed to both central changes (decreased SV from impaired myocardial function and increased venous pooling) and peripheral changes characteristic of aerobically inefficient muscle fibers (decreases in oxidative enzyme concentrations, mitochondria, and capillary density).22 Significant associations have been found between physical activity and physical environmental factors such as accessibility, esthetic attributes, and opportunities for activity within the general public.95 However, the influence of such factors on cardiovascular and pulmonary fitness of people with neurological disabilities has received little attention in the literature. For most individuals with neurological conditions, the existence of neuromuscular impairments confounds interpretation of Vo2peak testing. When people with an intact nervous system are tested, normal biomechanical efficiency is assumed; an impaired nervous system increases the complexity of physiological responses. Both primary effects of upper motor neuron damage (e.g., paralysis, incoordination, spasticity, sensory-perceptual disorders, balance disturbances) and secondary “peripheral” changes in skeletal muscle (e.g., gross muscular atrophy96 and changes in muscle fiber composition97) affect the response to exercise. As a result, people with neurological disabilities manifest not only metabolic but also biomechanical defficiencies, both of which contribute to reduction in functional capacity. Consequently, the decline in exercise capacity is greater than expected (e.g., in people with postpoliomyelitis syndrome, deterioration in Vo2peak over a 3- to 5-year period was 12% greater than the predicted decline98). Paresis reduces the pool of motor units available for recruitment during physical work,99 thereby reducing the metabolically active tissue and lowering the oxidative potential.100 In the case of stroke, an estimated 50% of the normal number of motor units are functioning,101 and a strong relationship between bilateral thigh muscle mass and Vo2peak has been reported.102 In addition, along with altered joint kinematics and decreased postural reactions,103 children with CP exhibit high levels of co-contraction (simultaneous contraction of agonist and antagonist muscle groups), which may prematurely induce skeletal muscle fatigue, further increasing the energy expenditure of walking and decreasing Vo2peak.104,105 Thus, when compared with able-bodied individuals, children with CP experience greater levels of fatigue at slower walking speeds.104,105 For people with postpoliomyelitis syndrome, muscle weakness of the lower extremities is strongly associated with energy expenditure of walking.49 In fact, when compared with healthy age- and sex-matched subjects, energy cost of walking is found to be significantly higher (40%) for people with postpoliomyelitis syndrome.49 Altered fiber composition and recruitment patterns of paretic muscle may also contribute to poor fitness.97,106 Skeletal muscles are composed of fibers that express different myosin heavy chain (MHC) isoforms. Slow (type I) MHC isoform fibers have higher oxidative function, are more fatigue resistant, and are more sensitive to insulin-mediated glucose uptake; fast (type II) MHC fibers are recruited for more powerful movements, are more reliant on anaerobic or glycolytic means of energy production, fatigue rapidly, and are less sensitive to the action of insulin.107 Although relatively equal proportions of slow and fast MHC isoforms are found in the vastus lateralis of healthy individuals,100 elevated proportions of the fast, more fatigable fibers that are less glucose sensitive have been found in the paretic leg of people after a stroke.97 Hence it is likely that reduced insulin sensitivity and increased use of the anaerobic processes during dynamic exercise at the level of the muscle contribute to reductions in Vo2peak. Furthermore, alterations in the structure of mitochondria100 and reduced activity of oxidative enzymes (e.g., succinate dehydrogenase)108 may contribute to the reduced oxidative capacity of paretic muscles. Cardiovascular comorbidities, prevalent in populations with neurological disorders, contribute to metabolic inefficiency. In fact, cardiovascular complications are the leading cause of death in persons with stroke,109 MS,110 and SCI.111 About 75% of patients who have had a stroke are hypertensive,112 and the same proportion of patients have underlying cardiovascular dysfunction.113 In fact, most persons who have had a stroke have atherosclerotic lesions throughout their vascular system,114 and a high correlation has been reported between the number and degree of stenotic lesions in the coronary and carotid arteries.115,116 The high prevalence of CAD in this population should not be surprising because stroke and cardiac disease share similar predisposing factors (e.g., older age, hypertension, diabetes mellitus, cigarette smoking, sedentary lifestyle, and hyperlipidemia) and pathogenic mechanisms (e.g., atherosclerosis).117 Metabolic syndrome is a usual construct in identifying patients at high risk for future vascular events (e.g., a second stroke, myocardial infarction).118 Metabolic syndrome refers to a constellation of markers of metabolic abnormalities (i.e., hypertension, abdominal obsesity, abnormal lipid profile) that interact to accelerate the progression of atherosclerosis and increase the risk of development of cardiovascular or cerebrovascular disease.119 The prevalence of metabolic syndrome in neurological populations is high. A retrospective study reported that about 61% of 200 patients in stroke rehabilitation met the criteria for the syndrome.120 Factors that elevate HR for a given Vo2, such as CAD, result in attainment of a peak HR (HRpeak) at a Vo2peak below that predicted for that individual. Cardiac dysfunction contributes to a lower aerobic capacity through two principal mechanisms: ischemia-induced reductions in ejection fraction and SV with exercise121 and chronotropic incompetence—the inability to increase HR in proportion to the metabolic demands of exercise.21 For persons who can attain HRmax within 15 bpm of the predicted maximum, limitations in exercise capacity probably do not have cardiovascular causes. Impaired peripheral blood flow also contributes to reduced cardiovascular fitness. Inadequate blood flow to the periphery impairs O2 transport and limits energy production in the working muscles, thereby compromising the ability to sustain physical activity. Both resting blood flow and postischemic reactive hyperemic blood flow have been found to be lower (approximately 36% less) in the paretic leg of people poststroke.122,123 In addition, despite near-normal (above 0.90) mean ankle brachial index values, arterial diameter has been found to be reduced poststroke.122 Potential mechanisms responsible for reduced blood flow on the hemiparetic side include altered autonomic function,124 enhanced sensitivity to endogenous vasoconstrictor agents,125 and altered histochemical and morphological features of the vascular network itself.126 However, the relative contribution of each of these factors is unknown. In addition, local metabolic mediators associated with changes in muscle fiber composition in the paretic limb (previously discussed) may contribute to impaired limb blood flow.100 Trauma to the spinal cord may disrupt the autonomic reflexes and sympathetic vasomotor outflow required for normal cardiovascular responses to exercise.127 As a result, reduced venous return and cardiac output (referred to as circulatory hypokinesis) impair delivery of O2 and nutrients to and removal of metabolites from working muscles, intensifying muscle fatigue.128 For people with paraplegia, an exaggerated HR response may occur during exercise in order to compensate for reduced SV. However, adrenergic dysfunction associated with lesions above the T1 sympathetic outflow prevent this compensatory mechanism,129 thereby increasing the risk of cardiovascular disease. Typically in able-bodied individuals the pulmonary system does not limit cardiopulmonary fitness, because the lungs of people without chronic health conditions have a large reserve.130 Nevertheless, at maximal workloads as much as 10% of Vo2max is needed to support the mechanical work of the diaphragm, accessory inspiratory muscles, and abdominal muscles.121 In contrast, people with neurological impairments may have limited O2 availability for exercise as a result of pathological conditions involving the pulmonary system, either as a direct complication of a neuromuscular condition (e.g., muscle weakness, impaired breathing mechanics) or as a result of cardiovascular dysfunction, comorbidities (e.g., chronic obstructive pulmonary disease), or lifestyle factors (e.g., physical inactivity, smoking habits).131,132 These impairments can reduce the ventilatory reserve, defined as the difference between the maximal available ventilation and the ventilation measured at the end of exercise.133 As previously mentioned, minute ventilation is closely associated with Vco2 during exercise. At peak exercise, a ratio of minute ventilation to Vco2 above between 35134 and 40135 indicates an abnormal ventilatory response. Neu and colleagues136 reported an 87% incidence of obstructive pulmonary dysfunction in patients with Parkinson disease, despite the finding that Vo2peak levels in this patient group tend to be in the normal range.137,138 For children with CP, reduced exercise capacity may be partly caused by respiratory muscle spasticity resulting in reduced breathing efficiency.105 In the case of stroke, pulmonary function is usually affected to only a modest extent, notwithstanding acute respiratory complications (e.g., pulmonary embolism, aspiration pneumonia).131 Impaired respiration may be attributed to cardiovascular dysfunction or lifestyle factors (e.g., physical inactivity, high incidence of smoking)139 or a direct result of the stroke, particularly brain stem stroke. The overwhelming fatigue felt by some persons after a stroke may be partly caused by respiratory insufficiency as manifested by low pulmonary diffusing capacity, decreased lung volumes, and ventilation-perfusion mismatching.140 Impaired breathing mechanics with restricted and paradoxical chest wall excursion and depressed diaphragmatic excursion have been also reported.131,141 Expiratory dysfunction appears to be related to the extent of motor impairment (e.g., hemiabdominal muscle weakness), whereas inspiratory limitations appear to be related to the gradual development of rib cage contracture.142 To summarize, a host of interacting factors are associated with abnormally low cardiopulmonary fitness in people with neurological disorders. Neuromuscular and respiratory dysfunctions are often superimposed on an already-compromised state as a result of comorbid cardiovascular disease and premorbid health- and lifestyle-related declines. Paresis and the subsequent reduction in lean muscle mass, changes in the muscle fiber phenotype, and increased reliance on anaerobic processes for energy production result in high metabolic costs of moving paretic limbs. As a consequence, cardiac reserves available for meaningful activity-level functions are limited, which in turn has a negative impact on participation-level functions. Collectively, impairments in the neuromuscular, cardiovascular, and pulmonary systems converge to promote a sedentary lifestyle and reduced health-related quality of life, which in turn leads to further inactivity and further reductions in cardiopulmonary fitness. The contribution of skeletal system impairments to this downward spiral has received little attention. Pang and colleagues143 studied the relationship between bone health and physical fitness in patients who had had a stroke and found a significant correlation between paretic femur bone mineral density and Vo2max. They concluded that further study is needed to determine the clinical implications of this finding. It is now apparent that healthy young and old individuals who begin participating in regular activity even after years of inactivity can enjoy greater health and fitness than those who remain sedentary.144 Training studies involving people with neurological disability, although limited in number and sample size and often lacking a control group, provide preliminary evidence of cardiopulmonary adaptations to physical work (Table 30-2). For example, for adolescents with chronic disabilities (including CP, SpinaBefida [SB], and SCI)91 and children with CP,145–148 cardiovascular fitness training is found to produce positive results on aerobic capacity and fitness. There is also growing evidence supporting the benefits of aerobic training in people with stroke of mild to moderate severity.149 Exercise training has also been found to improve the physical capacity of people with SCI.150,151 In the case of people with TBI, evidence regarding the effects of exercise training has been inconclusive.152 However, in people with neurological disorders, cardiovascular adaptations in response to aerobic training enhance metabolic efficiency, and neuromuscular adaptations in response to strength and gait training improve mechanical efficiency. The result is improved functional capacity with lowered energy costs of ADLs, enhanced fatigue resistance, and increased exercise tolerance (Figure 30-3). TABLE 30-2
Cardiovascular and pulmonary system health in populations with neurological disorders
Physiological responses to exercise
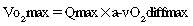
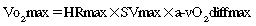
Measurement of cardiopulmonary fitness
Testing modality
Predicting maximal oxygen consumption with use of submaximal exercise tests
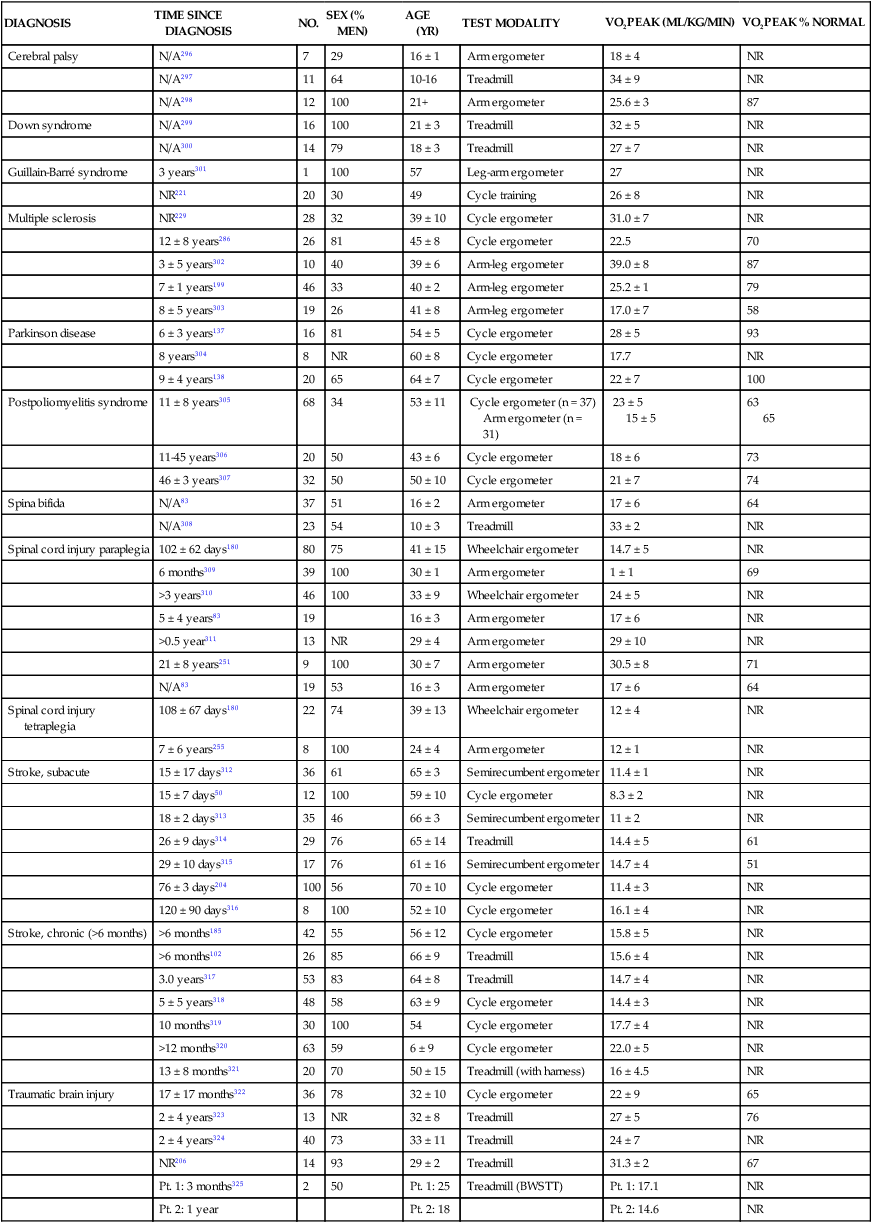
Fitness levels in populations with neurological disorders

DIAGNOSIS
TIME SINCE DIAGNOSIS
NO.
SEX (% MEN)
AGE (YR)
TEST MODALITY
VO2PEAK (ML/KG/MIN)
VO2PEAK % NORMAL
Cerebral palsy
N/A296
7
29
16 ± 1
Arm ergometer
18 ± 4
NR
N/A297
11
64
10-16
Treadmill
34 ± 9
NR
N/A298
12
100
21+
Arm ergometer
25.6 ± 3
87
Down syndrome
N/A299
16
100
21 ± 3
Treadmill
32 ± 5
NR
N/A300
14
79
18 ± 3
Treadmill
27 ± 7
NR
Guillain-Barré syndrome
3 years301
1
100
57
Leg-arm ergometer
27
NR
NR221
20
30
49
Cycle training
26 ± 8
NR
Multiple sclerosis
NR229
28
32
39 ± 10
Cycle ergometer
31.0 ± 7
NR
12 ± 8 years286
26
81
45 ± 8
Cycle ergometer
22.5
70
3 ± 5 years302
10
40
39 ± 6
Arm-leg ergometer
39.0 ± 8
87
7 ± 1 years199
46
33
40 ± 2
Arm-leg ergometer
25.2 ± 1
79
8 ± 5 years303
19
26
41 ± 8
Arm-leg ergometer
17.0 ± 7
58
Parkinson disease
6 ± 3 years137
16
81
54 ± 5
Cycle ergometer
28 ± 5
93
8 years304
8
NR
60 ± 8
Cycle ergometer
17.7
NR
9 ± 4 years138
20
65
64 ± 7
Cycle ergometer
22 ± 7
100
Postpoliomyelitis syndrome
11 ± 8 years305
68
34
53 ± 11
Cycle ergometer (n = 37)
Arm ergometer (n = 31)
23 ± 5
15 ± 5
63
65
11-45 years306
20
50
43 ± 6
Cycle ergometer
18 ± 6
73
46 ± 3 years307
32
50
50 ± 10
Cycle ergometer
21 ± 7
74
Spina bifida
N/A83
37
51
16 ± 2
Arm ergometer
17 ± 6
64
N/A308
23
54
10 ± 3
Treadmill
33 ± 2
NR
Spinal cord injury paraplegia
102 ± 62 days180
80
75
41 ± 15
Wheelchair ergometer
14.7 ± 5
NR
6 months309
39
100
30 ± 1
Arm ergometer
1 ± 1
69
>3 years310
46
100
33 ± 9
Wheelchair ergometer
24 ± 5
NR
5 ± 4 years83
19
16 ± 3
Arm ergometer
17 ± 6
NR
>0.5 year311
13
NR
29 ± 4
Arm ergometer
29 ± 10
NR
21 ± 8 years251
9
100
30 ± 7
Arm ergometer
30.5 ± 8
71
N/A83
19
53
16 ± 3
Arm ergometer
17 ± 6
64
Spinal cord injury tetraplegia
108 ± 67 days180
22
74
39 ± 13
Wheelchair ergometer
12 ± 4
NR
7 ± 6 years255
8
100
24 ± 4
Arm ergometer
12 ± 1
NR
Stroke, subacute
15 ± 17 days312
36
61
65 ± 3
Semirecumbent ergometer
11.4 ± 1
NR
15 ± 7 days50
12
100
59 ± 10
Cycle ergometer
8.3 ± 2
NR
18 ± 2 days313
35
46
66 ± 3
Semirecumbent ergometer
11 ± 2
NR
26 ± 9 days314
29
76
65 ± 14
Treadmill
14.4 ± 5
61
29 ± 10 days315
17
76
61 ± 16
Semirecumbent ergometer
14.7 ± 4
51
76 ± 3 days204
100
56
70 ± 10
Cycle ergometer
11.4 ± 3
NR
120 ± 90 days316
8
100
52 ± 10
Cycle ergometer
16.1 ± 4
NR
Stroke, chronic (>6 months)
>6 months185
42
55
56 ± 12
Cycle ergometer
15.8 ± 5
NR
>6 months102
26
85
66 ± 9
Treadmill
15.6 ± 4
NR
3.0 years317
53
83
64 ± 8
Treadmill
14.7 ± 4
NR
5 ± 5 years318
48
58
63 ± 9
Cycle ergometer
14.4 ± 3
NR
10 months319
30
100
54
Cycle ergometer
17.7 ± 4
NR
>12 months320
63
59
6 ± 9
Cycle ergometer
22.0 ± 5
NR
13 ± 8 months321
20
70
50 ± 15
Treadmill (with harness)
16 ± 4.5
NR
Traumatic brain injury
17 ± 17 months322
36
78
32 ± 10
Cycle ergometer
22 ± 9
65
2 ± 4 years323
13
NR
32 ± 8
Treadmill
27 ± 5
76
2 ± 4 years324
40
73
33 ± 11
Treadmill
24 ± 7
NR
NR206
14
93
29 ± 2
Treadmill
31.3 ± 2
67
Pt. 1: 3 months325
2
50
Pt. 1: 25
Treadmill (BWSTT)
Pt. 1: 17.1
NR
Pt. 2: 1 year
Pt. 2: 18
Pt. 2: 14.6
NR
Impact of low fitness levels on health of people with neurological disorders
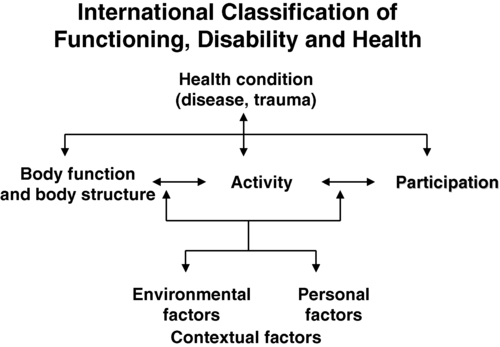
Factors affecting fitness levels in people with neurological disorders
Personal and environmental contextual factors
Age
Sex
Lifestyle factors
Environmental factors
Health condition
Neuromuscular system
Cardiovascular system
Pulmonary system
Adaptive responses to aerobic training in populations with neurological disorders

DIAGNOSIS
TRAINING MODE
NO.
PROGRAM WEEKS
FREQUENCY (TIMES PER WEEK)
DURATION (MIN)
INTENSITY
% VO2PEAK CHANGE
Cerebral palsy
Uphill walking296
E: 7
12
3
20-22
65%-75% HRmax
E: +19
C: 6
C: +0.6
Guillain-Barré syndrome
Arm-leg ergometer301
E: 1
16
3
20
75%-85% HRmax
E: +9
Cycle training221
E: 20
12
3
NR
NR
E: +16
Multiple sclerosis
Arm-leg ergometry199
E: 21
15
3
30
60% Vo2peak
E: +22
C: 25
C: +1
Leg cycle ergometer303
E*: 11
8
3
60
60%-80% maximum work rate
Postpoliomyelitis syndrome
Cycle ergometer167
E: 16
16
3
15-30
70% HRmax
E: +15*
C: 21
C: +4
Arm ergometer306
E: 10
16
3
20
70%-75%
E: +19
C: 10
HRR or 50-60 rpm or RPE6-20 = 13
C: −1
E1: Aerobic exercise, hospital231
E1: 15
8
3
30
50%-70% of Vo2peak
E1: +23
E2: Aerobic exercise, home
E2: 13
E2: +7
Spinal cord injury—C7-T12
FES-assisted rowing166
E: 6
6
3
30
75%-80% Vo2peak
E: +11
Circuit training262
E: 7
16
3
40-45
50%-60% 1 RM
E: +10
Spinal cord injury—tetraplegia
FES-assisted ergometry164
E: 18
12-16
3
30
0-31 W
E: +23
Arm ergometer255
E: 8
8
3
30
50%-60% of HRR or 60 rpm
E: +94
Stroke—subacute
Stationary bicycle204
E: 44
12
3
20-30
40 rpm
E: +9
C: 48
C: +0.5
Treadmill50
E: 6
26
5
20
NR
E: +35
C: 6
C: +1
Cycle ergometer312
E: 23
3.3-4.1
3
30
50%-75% of Vo2peak
E: +13
C: 22
C: +8
Stroke—chronic
Cycle ergometer207
E: 37
26
3
10-20
40%-50% HRR
E: +18
C: 24
C: −3NS
Cycle ergometer203
E: 24
8
2
20
50%-60% HRR
E: +13
C: 24
C: 0NS
Treadmill with harness support197
E: 26
26
3
40
60%-70% HRR
E: +15
C: 20
C: −3NS
E1: Moderate intensity326
E1: 18
14
3
30-60
E1: 50%-69% HRR
E1: +4NS
E2: Low intensity326
E2: 19
E2: <50% HRR
E2: +6NS
C: 18
C: −3NS
Treadmill327 (plus strength training)
E: 14
12
5
90
E: 80% HRmax
E: +19
Treadmill321
E: 20*
4
2-5
NR
80%-85% HRmax or RPE of 17
Immediate: +6
Delayed: +6
Stationary bicycle185
E: 19
10
3
30
50-70 rpm
E: +13
C: 23
C: +1
Aerobic exercise155
E: 29
12
3
30
HR = (HR at RER = 1) -15
E: +8
Treadmill40
E: 23
26
3
20
<60% HRR
E: +10
Aerobic exercise328
E: 32
19
3
60
<80% HRR
E: +9
C: 31
C: +1
Water based163
E: 7
8
3
30
<80% HRR
E: +23
C: 5
C: +3
Traumatic brain injury
Low-intensity aerobic exercises324
E: 40
16
3
15-20
“Low”
E: +3
Circuit training206
E: 14
16
3
45
70% of Vo2peak
E: +15
Treadmill, walking, jogging249
E: 32
12
3
60
“Moderate intensity”
E and C: +14
C: 30
BWSTT325
E: 2
Pt. 1: 11
2-3
17-32
Pt. 1: 60%-85% HRmax
Pt. 1: +24
Pt. 2: 15
Pt. 2: <50% HRmax
Pt. 2: +16 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cardiovascular and pulmonary system health in populations with neurological disorders
Only gold members can continue reading. Log In or Register to continue