Chapter 57 Cancer Rehabilitation
Epidemiology
Cancer is a prevalent condition that becomes increasingly common with advancing age. Just under 1.5 million new cancers were diagnosed within the United States in 2009, and more than 560,000 people died of cancer.119 Cancer causes one in four deaths, and is second only to heart disease as the leading cause of mortality in the United States.119 Roughly 76% of all cancers occur in patients 55 years of age and older.7 Men are more commonly affected by cancer (excluding basal and squamous cell cancers of the skin), with a lifetime risk in the United States of one in two. The lifetime risk in women is one in three.7 Many cancers could be prevented through behavioral modification. One third of all cancer deaths are related to obesity, physical inactivity, and other lifestyle factors.7 Only 5% to 10% of cancers are hereditary and directly related to aberrantly expressed or regulated genes.
Survivorship
Cancer 5-year survival rates are increasing as a result of a variety of factors, including successful early detection efforts, improved multimodality treatments, and expansion of the chemotherapeutics and biopharmaceuticals available to treat patients with metastatic disease. Sixty-six percent of adult cancer patients live 5 years beyond diagnosis.107 These numbers do not accurately reflect current trends because the statistics pertain to patients who were treated 5 years ago, and the standards of care for many cancers have changed. The prevalence of cancer survivors will increase, given the anticipated persistence of factors responsible for current survivorship trends.107 First, the aging of the population will produce an increase in the incidence of age-related cancers such as colon, breast, and prostate cancer. Second, early detection efforts are being aggressively funded and implemented. We can expect that more and more cancers will be identified at early, curable stages. Last, clinical research continues to refine strategies for delivering established and novel anticancer therapies. During the past several years we have witnessed an unprecedented influx of targeted biopharmaceuticals into the cancer treatment arsenal. Collectively these efforts have consistently produced incremental outcome improvements. It is reasonable to assume that this trend will continue to benefit both patients who are cured and those who are living with cancer.
Disease Considerations
Prognosis and Metastatic Spread
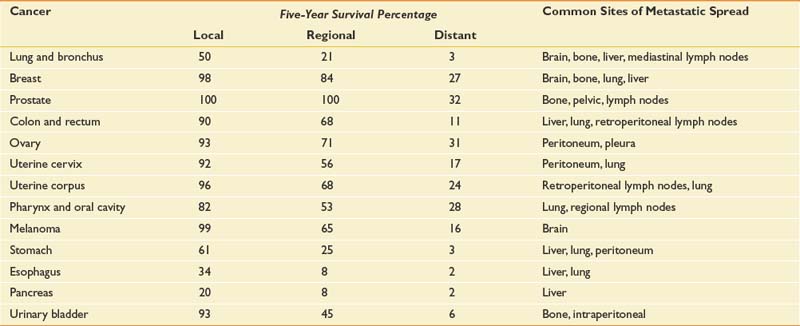
Table 57-1 presents 5-year survival statistics collected between 1996 and 2004 for different cancers.107,119 The implications of regional and distant spread at the time of diagnosis vary considerably by cancer type. For example, prostate cancer patients enjoy an excellent prognosis when their cancer is detected at the local or regional level, with virtually 100% 5-year survival. However, only 21% of lung cancer patients with regional spread are alive at 5 years. It is critical to bear in mind that survival statistics are mean values, with potentially wide confidence intervals that provide crude estimates—but are potentially imprecise when applied to individual patients.
This information informs rehabilitation goal setting, determines the level of emphasis placed on symptom-oriented versus disease-modifying treatments, and allows for the assessment of patients’ unduly optimistic or grim expectations. Table 57-1 also lists common sites of metastases for prevalent malignancies. Understanding patterns of metastatic spread can help clinicians focus the search for metastases. Lung, breast, colon, and melanoma commonly spread to the brain. Regular neurologic screening examinations should therefore be incorporated into posttreatment, surveillance care. Prostate, breast, and lung cancer commonly produce bone metastases. Musculoskeletal pain in these cancer populations can be due to the primary or secondary consequences of bony disease and should trigger an appropriate evaluation.
Phases of Cancer
For rehabilitation purposes, cancer can be divided into several distinct stages. This approach calls clinical attention to certain nodal points along the disease trajectory that should trigger a revaluation of functional deficits, reinvolvement of rehabilitation services, and redefinition of functional goals. Five distinct stages of malignant disease—initial diagnosis and treatment, surveillance, recurrence, temporization, and palliation—were initially outlined in a model proposed by Gerber et al.80 Phases of cancer should determine rehabilitation goals with interphase transitions mandating reassessment of goals. Attention to cancer phases ensures that significant shifts in prognosis and treatment requirements inform rehabilitative efforts.
Constitutional Symptoms
Fatigue
Fatigue is the most common symptom experienced by cancer patients.180 The prevalence of fatigue ranges from 70% to 100%, contingent on the type and stage of cancer. It is also related to whether patients are receiving anticancer treatments.180,219 A majority of patients in active treatment rate their fatigue as ‘‘severe’’ or 7 or more on an 11-point numerical rating scale.102 Because fatigue is an inherently subjective, definitions of fatigue understandably differ. The National Comprehensive Cancer Network defines CRF as: “an unusual, persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning.”181 Experts concur that fatigue reduces the energy, mental capacity, functional status, and psychologic resilience of cancer patients.181 The novel International Classification of Diseases, 10th edition criteria for CRF, listed in Box 57-1, reflect this consensus.
BOX 57-1 International Classification of Diseases, 10th edition, Criteria for Cancer-Related Fatigue
A discrete source of fatigue can be identified in some patients, leading to effective treatment and symptom reversal. More often the responsible mechanisms are multifactorial. Box 57-2 lists possible contributing factors. Anemia has typically received the greatest amount of attention as a source of fatigue, but this focus has shifted in recent years. Previous interest was due, in part, to the high prevalence of fatigue among cytopenic cancer patients receiving chemotherapy (38% to 86%),10,254 and to reports that the onset and severity of fatigue paralleled reductions in serum hemoglobin.34 More recent, comprehensive data demonstrate that the time course of fatigue differs from fluctuations in blood counts and that normalization of hemoglobin levels often fails to reduce fatigue. No specific decrement or increment in hemoglobin levels has been definitely associated with meaningful changes in patients’ quality of life (QOL). Of greater concern are the findings that patients receiving erythropoiesis-stimulating agents have an elevated risk of thromboembolism, that several randomized trials have demonstrated decreased survival times in cancer patients receiving erythropoiesis-stimulating agents, and that two randomized trials have demonstrated poorer “locoregional” control or progression-free survival in cancer patients receiving these agents.18,212
Despite this, erythropoiesis-stimulating agents continue to be used in the treatment of anemia related to cancer treatment. A case has been made for initiating therapeutic doses in appropriate patients receiving anticancer treatment. The American Society of Clinical Oncology/American Society of Hematology guidelines endorse the use of 10 g/dL as the threshold hemoglobin value to recommend initiating an erythropoiesis-stimulating agent.212 Starting doses should be determined by the package insert of the specific agent. Continuing erythropoiesis-stimulating agents beyond 6 to 8 weeks in nonresponding patients does not appear to be effective. Iron stores should be monitored and supplemented as required for patients treated with erythropoiesis-stimulating agents. Patients who have poor responses to epoetin therapy, intensely symptomatic anemia, hemoglobin levels less than 9 g/dL, or economic constraints to erythropoiesis-stimulating agents might require red blood cell transfusion.
Deconditioning secondary to inactivity is common among cancer patients.49 If deconditioning does not initiate fatigue, it frequently aggravates fatigue arising from other sources. Mood-related factors such as anxiety and depression are also prevalent among cancer patients. Thirty percent of patients develop clinical depression after a cancer diagnosis.69 Centrally acting medications should be carefully reviewed in patients complaining of fatigue. A reduction or withdrawal trial of nonessential drugs can identify those producing fatigue.181 Medications that commonly produce fatigue include opioids, benzodiazepines, antiemetics, antihistamines, tricyclic antidepressants, anticonvulsants (e.g., carbamazepine, gabapentin, and oxcarbazepine), thalidomide, and α2-adrenergic agonists (e.g., tizanidine).
In the absence of a discernible etiology, CRF might be associated with elevated cytokine levels. Cytokines such as tumor necrosis factor, interleukin-1, and interleukin-6 have been implicated in CRF.87 The mechanism(s) by which elevations in circulating cytokine levels produce fatigue, however, and whether they are elaborated by host or tumor cells, remains unclear. Cytokine antagonists are not recommended at this time for the treatment of CRF.
When potentially reversible sources of fatigue (see Box 57-2) have been ruled out or definitively addressed, symptom-oriented fatigue management is indicated. The National Comprehensive Cancer Network endorses a multimodal approach that includes medications, exercise, psychologic interventions, and improved sleep hygiene as offering the greatest likelihood of success.181 The use of aerobic exercise to reduce CRF is discussed at length in the “Aerobic Conditioning” section of this chapter.
Methylphenidate has been used most extensively to treat fatigue in cancer patients. Four open-label studies in mixed cancer cohorts have demonstrated reduced fatigue with methylphenidate.23,91,105,224 A fifth open-label pilot study combining exercise and methylphenidate also reported benefit.232 However, results from five randomized, controlled, double-blinded studies conflict. Two studies published by Lower et al.150,151 detected reduced fatigue in patients who had completed cytotoxic chemotherapy. In three additional trials in mixed brain and breast cancer populations, however, methylphenidate did not differ from placebo in reducing CRF.25,27,155 These inconsistencies could be due to different maximal doses, trial duration, and inclusion criteria. Currently it is reasonable to trial methylphenidate at a starting dose of 5 to 10 mg/day. Dose-limiting toxicities associated with methylphenidate include anorexia, insomnia, anxiety, confusion, tremor, and tachycardia. Dose titration continues gradually until a therapeutic response is achieved or adverse side effects preclude further dose escalation. Doses greater than 60 mg/day are rarely required.
Modafinil has been less extensively studied in two open-label trials with disparate study populations. Both breast cancer survivors and patients with brain tumors reported less fatigue while taking modafinil.122,177 Modafinil is generally tolerated with few side effects (e.g., headache, anxiety, nausea). When present, these symptoms are rated as mild and resolve on discontinuation. Modafinil therapy can be initiated at 100 to 200 mg/day and titrated to a maximal dose of 400 mg/day.
Pain
The prevalence of cancer-related pain is 28% among patients with newly diagnosed cancer,269 50% to 70% among patients receiving antineoplastic therapy,198,199 and 64% to 80% among patients with advanced disease.29,63,257 Adequate pain control is an absolute requisite for successful rehabilitation. Cancer patients generally experience multiple concurrent pain syndromes. Thorough evaluation requires assessment of all relevant pain etiologies and pathophysiologic processes. Pain control might require the integrated use of anticancer treatments, agents from multiple analgesic classes, interventional techniques, topical agents, manual approaches, and modalities.
The unique disease context in which cancer pain develops distinguishes it from many other pain-associated diagnoses managed by physiatrists. Considerations in cancer pain management are listed in Box 57-3 and explained below. One of the most salient features of cancer pain management is the reliance on high-dose opioid therapy. The doses required by many cancer patients can extend far beyond the conventional levels used by physiatrists. Fifteen percent of a cohort of stage IV pancreatic cancer patients required more than the daily equivalent of 5 g of parenteral morphine.72 However, extensive international literature and multiple guidelines resoundingly endorse this approach.12,64,168,187
The majority of cancer pain is due to tumor effects. For this reason, disease-modifying, anticancer therapy plays a critical role in pain management. For example, a single radiation fraction of 8 Gy offers a definitive and effective means of controlling pain associated with symptomatic and uncomplicated bone metastases.277 Cancer progression frequently causes pain to worsen, and escalating analgesic requirements should be anticipated.72 Cancer-related depression, anxiety, and existential distress can exacerbate patients’ pain experience.246 For this reason, contributing psychiatric factors should be addressed.
Acute Pain
Acute pain after surgery or radiation therapy can be successfully treated using conventional algorithms for acute postoperative pain.3 Nerves are frequently severed, compressed, or stretched during tumor resection, making it possible for neuropathic pain to be a major factor during the postoperative period. Neural compromise contributes significantly to postmastectomy and postthoracotomy pain syndromes. Adjuvant analgesics (e.g., gabapentin) should be initiated when a neurogenic contribution to the pain is suspected. As with all postoperative pain that impedes function, aggressive opioid-based and antiinflammatory analgesia should be considered. Acute pain control allows movement and limits immobility. This is particularly important in cancer patients who face the debilitating effects of chemotherapy or radiation therapy shortly after surgery.
To allow patients whose cancers eventually recur or progress to benefit from opioid rotation, opioid use should be confined to the “immediate-” and “sustained-” or “continuous-release” formulations of a single drug. The dose threshold for switching opioids because of lack of efficacy in patients with poor prognoses should be high. In this way, patients’ exposure can be restricted to a limited number of opioids, allowing them to benefit from opioid rotation in the late stages of disease.112,166
Acute pain can also complicate the administration of chemotherapy, hormonal therapy, and irradiation. Most of the associated pain syndromes are transient but can produce intense discomfort that warrants aggressive analgesia. Acute pain syndromes associated with cancer therapy include paclitaxel-related arthralgias and myalgias,217 bisphosphonate-related bone pain,104 radiation mucositis,211 steroid pseudorheumatism (after withdrawal of corticosteroids),216 intravesicular Bacillus Calmette Guerin (BCG–induced cystitis, hepatic artery infusion pain,124 bone pain associated with colony-stimulating factor (CSF) and granulocyte macrophage CSF administration,266 and radiopharmaceutical-induced pain.
Chronic Pain
Chronic cancer-related pain can arise from visceral or neural structures but is most commonly associated with bone metastases.145 Bone metastases occur in 60% to 84% of patients with solid tumors. Pain intensity does not correlate with the number, size, or location of bone metastases. Pain intensity also does not correlate with tumor type because 25% of patients with bone metastases report no pain.210 Bone pain is particularly relevant to physiatrists because recruiting muscles that act on or loading affected structures can precipitate severe pain. Too often the excellent pain control achieved while patients remain in bed proves inadequate when they begin to transfer and ambulate. As mentioned above, bone pain responds well to local irradiation.277
Nonsteroidal Antiinflammatory Drugs for Bone Pain
Pharmacologic interventions reduce the intensity of bone pain. Prostaglandins have been implicated in pain associated with lytic bone metastases.167 Blockade of prostaglandin synthesis is likely the principal mechanism by which nonsteroidal antiinflammatory drugs (NSAIDs) alleviate bone pain.221 NSAIDs are considered first-line therapy for bone pain, and a trial is warranted unless contraindicated. Patients’ limited prognoses and the intensity of their suffering might eclipse cyclooxygenase (COX)-2 inhibitors’ worrisome cardiovascular risk profile. Although caution should be exercised, the significant potential benefits of COX-2 inhibitors outweigh their risks in many cancer patients with thrombocytopenia and/or gastropathy. Currently celecoxib is the only COX-2 inhibitor for oral use that remains available on the U.S. market.
COX nonselective inhibitors offer comparable or greater pain relief but a less desirable toxicity profile.223 Choline magnesium trisalicylate causes less inhibition of platelet aggregation than other COX nonselective inhibitors, but did not statistically outperform placebo when trialed in cancer-related bone pain.121 COX nonselective inhibitors with less desirable toxicity profiles have proven more effective. Several placebo-controlled, randomized trials found that ketoprofen reduced cancer pain to a greater extent than either codeine or morphine.249 Dosing NSAIDs for bone pain is no different from using them at antiinflammatory doses for pain of other etiologies.
Adjuvant for Bone Pain
Adjuvant and opioid analgesics can augment NSAID-related control of bone pain. A study found corticosteroids to be beneficial in relieving cancer pain,24 and extensive anecdotal experience supports their use. The toxicity profile of corticosteroids includes edema, bone demineralization, immunosuppression, and myopathies. This mandates that they be used transiently and rapidly tapered, except for patients in whom sustained analgesic benefit justifies the associated toxicity risk.
Use of calcitonin for bone pain is discouraged because of the weak supportive evidence and rapid tachyphylaxis.158,167 Evidence supporting the use of parenteral bisphosphonates in the management of bone pain is more robust.54,167,267 Aminobisphosphonates appear to have greater effectiveness in reducing bone pain than nonaminobisphosphonates (such as clodronate), and are preferred for patients with high pain scores. Effective doses include 30 to 90 mg of intravenous pamidronate every 4 weeks, 4 mg of intravenous zoledronic acid every 3 weeks, and 1600 mg of oral clodronate daily. Opioids enhance analgesia afforded by NSAIDs and can reduce the doses required for adequate pain relief.242
Opioids
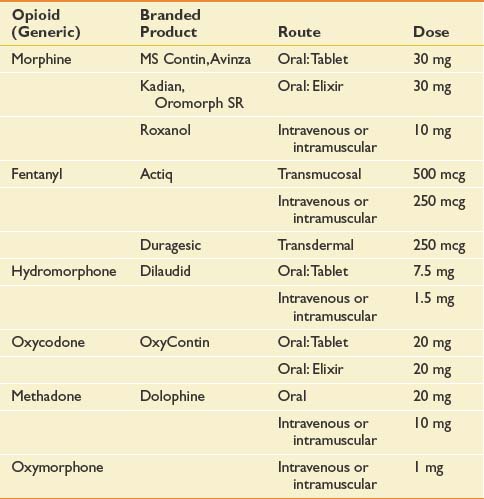
As previously mentioned, opioid-based pharmacotherapy is the current standard of care for the management of moderate to severe cancer pain, irrespective of its etiology.12,64,168,185 Opioid use should be restricted to pure μ-receptor agonists. Many μ-receptor agonists are commercially available in the United States. Those most commonly used in cancer pain management include morphine, hydromorphone, oxycodone, oxymorphone, fentanyl, and methadone. Opioid analgesic requirements change over time depending on whether a patient’s cancer progresses or responds to treatment. Ongoing dose adjustment maximizes pain control while reducing the incidence of side effects. The dominant paradigm for opioid administration has a well-established track record and has been reiterated by many experts in the field with few changes over the past.72,115,145
Opioid Conversion
Significant intraindividual variations in response to different opioids have long been recognized and are now presumed to result from genetically determined differences in pharmacokinetics and pharmacodynamics.75,214 An alternative opioid should be considered when an “adequate” trial of a particular agent has failed to achieve an acceptable decrement in pain intensity or has engendered refractory and untenable side effects. An adequate opioid trial in the cancer patient can entail use of high doses (e.g., >1 g/day intravenous morphine sulfate). Opioid dose conversion requires calculation of the equianalgesic dose of the novel agent (Table 57-2) and reduction by 50% for incomplete cross-tolerance. Incomplete cross-tolerance describes the property of opioids to induce analgesic tolerance with sustained high-dose opioid exposure. Tolerance is usually considerably lower to a novel agent. For this reason, patients often experience greater sedation and needless side effects when exposed to 100% of the equianalgesic dose. Reductions of 50% provide better estimates of the minimal effective opioid dose. If patients are being converted from methadone, reductions of 80% to 90% of the equianalgesic dose have been recommended because of methadone’s long half-life.274 Opioid conversions are based on imperfect dose equivalencies. Providing patients with liberal access to rescue doses is critical during the conversion period to avoid precipitation of pain crises.
Invasive and Intraspinal Analgesic Approaches
As mentioned previously, permanent ablation of central afferent tracts becomes tenable in the context of advanced cancer, and has been used with considerable success.36,88,255,272 More discrete neural blockade can effectively reduce pain transmitted by one or several adjacent peripheral nerves. Intercostal, paravertebral, genitofemoral, ilioinguinal, and trigeminal nerve blocks can afford dramatic relief and reduce analgesic requirements. Nociceptive impulses of visceral origin can be blocked by ablation of sympathetic ganglia. Celiac plexus blockade affords excellent relief of visceral cancer pain.67 Intraspinal opioid administration can reduce dose requirements and associated side effects.243 The potential benefits, however, must be weighed against the added cost, required maintenance, and risk of infection. Despite efforts to demonstrate cost savings through the use of implantable intrathecal opioid delivery systems,95 these devices are not widely used.
Impairments in Cancer
Impairments Caused by Tumor Effects
Bone Metastases
Bone metastases are an important source of cancer-related impairment and a critical consideration in rehabilitation.43 Surgical stabilization of acute or impending fractures produces impairments that warrant physiatric attention. Greater challenges arise when bone metastases produce severe, function-limiting pain or pose an uncertain fracture risk during therapeutic exercise. Bone metastases are highly prevalent because bone is the most common site of metastatic spread, and osseous lesions complicate the most frequently occurring cancers: lung, breast, and prostate. Thyroid cancer, lymphoma, renal cell carcinoma, myeloma, and melanoma also commonly spread to bone. Between 60% and 84% of patients with solid tumors will develop bone metastases.146,210 Management of bony metastatic pain is discussed in the preceding section on chronic pain. Of greatest physiatric concern are lesions involving the spine and long bones. These structures are critical for weight-bearing and mobility, and are the most prone to fracture. Bone metastases are managed with medications, radiopharmaceuticals, orthoses, radiation therapy, and/or surgical stabilization. The choice of intervention(s) will depend on lesion location, degree of associated pain, presence or risk for fracture, radiation responsiveness, and related neurologic compromise. The overall clinical context (e.g., prognosis, severity of medical comorbidities, and operative risk) must also be taken into consideration. Most patients with unfractured bony lesions can be treated nonoperatively with systemic therapy and radiation.
Bisphosphonates are the primary medications used to manage bone metastases. Use of these agents relieves pain and mitigates the spread and progression of bone metastases. Bisphosphonates are generally delivered parenterally. Bisphosphonates can reduce the risk of vertebral fracture (odds ratio, 0.69), nonvertebral fracture (odds ratio, 0.65), and hypercalcemia (odds ratio, 0.54).215 Bisphosphonates also significantly increase the time to first skeletal event after the initial detection of osseous metastases. Current evidence supports the empiric initiation of bisphosphonates in patients with bone metastases. Radiopharmaceuticals such as strontium-99 are predominantly used to manage severe, refractory pain associated with widely disseminated bone metastases. Drawbacks to radiopharmaceuticals include prolonged bone marrow suppression and potentially severe pain flares after administration.
Radiation delivered to bone metastasis offers an effective means of rapidly achieving local control of pain and tumor growth. Palliative radiation was formerly delivered in 10 fractions of 300 cGy. However, single fractions of 8 Gy also effectively alleviate pain.277 At present the protocols in use range between these extremes, with the choice of dose and schedule being heavily influenced by individual patient factors and institutional culture. Radiation can be delayed after surgical stabilization. It is an important adjunctive treatment, however, because it suppresses tumor growth in areas where surgical management could have distributed microscopic emboli.
Painful osteolytic lesions are predominantly responsible for pathologic fractures. The incidence of pathologic fracture among all cancer types is 8%.220 Breast carcinoma is responsible for roughly 53% of these. Other solid tumors associated with pathologic fractures are kidney, lung, thyroid cancer, and lymphoma. Sixty percent of all long bone fractures involve the femur, with 80% of these located in the proximal portion.210
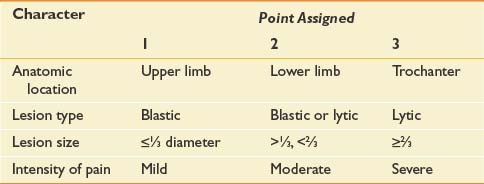
Management of osseous metastases that present a risk of fracture remains a source of clinical uncertainty. Precise quantification of fracture risk has been a persistent challenge in orthopedic oncology. Table 57-3 outlines Mirels’s proposed rating system for calculating fracture risk, whereby specific attributes are ascribed points.170 Neither this nor any other approach based on retrospective review has been adequately validated in clinical practice.
Pathologic fractures are generally managed through well-established surgical algorithms. Four main goals direct surgical management of pathologic fractures: pain relief, preservation or restoration of function, skeletal stabilization, and local tumor control.98 The general indications for surgery are life expectancy of more than 1 month with fracture of a weight-bearing bone, and more than 3 months for fracture of a non–weight-bearing bone. Internal fixation and prosthetic replacements with polymethylmethacrylate are the most effective ways of relieving pain and restoring function in patients with pathologic fractures.98 These procedures allow immediate weight-bearing. Intraoperative resection removes residual tumor that would impede bony healing, but healing rates are low after pathologic fractures. One review of 123 patients reported a 35% incidence of fracture healing.74
Fractures of the pelvis are generally treated conservatively, unless pain persists after radiation or they involve the acetabulum. In the latter case, patients are generally surgically reconstructed with screws or pins, and with an acetabular component. Vertebral fractures that are not associated with neurologic compromise are generally treated conservatively with radiation and bracing. Operative decompression and stabilization might be indicated for persistent pain refractory to aggressive analgesic therapy. Vertebroplasty can be considered for patients who are not at risk of tumor displacement into the spinal canal and associated myelopathy. Two large, recent randomized trials, however, have failed to demonstrate benefit in compression fractures related to osteoporosis.26,123 These results have raised skepticism regarding vertebroplasty’s benefit in cancer.
Brain Tumors: Primary and Metastases
Brain metastases occur in 15% to 40% of cancer patients, accounting for 200,000 new cases per year in the United States.78 They are the most common intracranial tumors.193 The incidence has increased in recent years, presumably as a result of prolonged patient survival and better early detection of small tumors through superior imaging modalities.66 Lung cancer is the most common primary source of brain metastases. As many as 64% of patients with stage IV lung cancer develop metastases.227 Breast cancer is the second most common source, followed by melanoma, with 2% to 25% and 4% to 20% of patients developing brain metastases, respectively.227 Brain metastases from colorectal cancers, genitourinary cancers, and sarcomas occur with considerably less frequency (1%).263 The distribution of metastases reflects cerebral blood flow, with 90% situated in the supratentorial region and 10% in the posterior fossa.263 Brain metastases are multiple in approximately 50% to 75% of cases.
Presentation
Lung cancer and melanoma often produce multiple metastases, whereas breast, colon, and renal cancer more commonly generate single lesions.263 Presenting symptoms at the time of diagnosis with brain metastasis, in order of decreasing frequency, are as follows (patients can have more than one): headache, 49%; mental disturbance, 32%; focal weakness, 30%; gait ataxia, 21%; seizures, 18%; speech difficulty, 12%; visual disturbance, 6%; sensory disturbance, 6%; and limb ataxia, 6%.201 Neurologic examination shows the following clinical signs at presentation: hemiparesis, 59%; impaired cognitive function, 58%; hemisensory loss, 21%; papilledema, 20%; gait ataxia, 19%; aphasia, 18%; visual field cut, 7%; and limb ataxia, 4%.201
Treatment
Corticosteroids are the first-line treatment, with dexamethasone being the drug of choice. By virtue of their ability to reduce peritumoral edema, corticosteroids reverse local brain compression and associated deficits. Treatment generally involves whole brain radiation therapy with stereotactic radiosurgery or surgical resection via craniotomy.125 Adjunctive chemotherapy can be used, contingent on patient performance status, type of cancer, and previous exposure to antineoplastics. Although seizures occur in 25% of patients with brain metastasis, studies and a metaanalysis have failed to show that antiepileptic drugs reduce their incidence.83,84
Prognosis
Untreated patients with brain metastases have a median survival of 1 to 2 months.154 Analyses performed by the Radiation Therapy Oncology Group that span multiple trials have produced a three-tiered classification scheme that predicts survival.77 Patients with the best prognoses (class 1), mean 7.1 months, had Karnofsky performance status (KPS) more than 70, age less than 65 years, controlled primary tumor, and no extracranial metastases. Patients with intermediate prognoses (class 2), mean 4.2 months, had KPS more than 70, with at least one of the following factors: more than 65 years of age, uncontrolled primary tumor, or systemic disease. Patients with poor prognoses (class 3), mean 2.3 months, had KPS less than 70. Although mean survival is less than 1 year for patients whose brain metastases are treated, the distribution is skewed, with some patients surviving more than 2 years with good functional preservation and QOL.
The rehabilitation needs of patients with brain metastases are determined by baseline functional status, prognoses, location and number of metastases, and antineoplastic treatment plan. The tremendous heterogeneity in the severity and type of associated impairments defies the formulation of a uniform algorithm. Cancer patients should be assessed on an individual basis using an approach analogous to that applied to patients with ischemic or traumatic intracranial lesions.
Epidural Spinal Cord Compression
Malignant spinal cord compression (SCC) occurs in up to 5% of patients.42 In contrast to brain metastases, which involve the brain parenchyma, most symptomatic tumors compress the spinal cord or cauda equina from the epidural space.200 Epidural lesions generally arise from vertebral metastases and rarely breach the dura.94 Invasion of the dural space accounts for only 5% of neoplastic SCC, and is due to either growth of tumor along the spinal roots or hematogenous spread to the cord.44,209 The cancers that most commonly cause SCC are those that produce vertebral metastases (e.g., breast, lung, myeloma, and prostate).17,276
Presentation
Pain is by far the most common initial (94%) and presenting (97% to 99%) symptom of malignant SCC.14,200 Radicular pain is present in 58% of patients at the time of diagnosis.14 Pain associated with SCC is generally exacerbated when supine or by coughing, sneezing, or the Valsalva maneuver. If malignant SCC is detected when pain is the only symptom, efforts to preserve function through surgical decompression or radiation therapy have high success rates.200 Unfortunately, this is rarely the case. Reports of symptom prevalence when the diagnosis of malignant SCC is eventually made are remarkably consistent. Weakness is present in 74% to 76% of patients, autonomic dysfunction in 52% to 57%, and sensory loss in 51% to 53%.81,200 The thoracic spine is the most common site of epidural SCC, followed by the lumbosacral and cervical spine in a ratio of 4:2:1.200
Diagnosis and Treatment
Magnetic resonance imaging (MRI) is the procedure of choice to evaluate the epidural space and spinal cord.251 MRI allows rapid evaluation of the entire spine with sagittal views. Computed tomography (CT) scans are helpful if there is an absolute contraindication to MRI, or if SCC is related to tumor encroachment through the foramina.
Prognosis
Tumors that cause rapid progression of neurologic deficits are associated with poorer functional outcomes after decompression.81 In general, patients remain ambulatory if able to walk at the time of definitive treatment. Motor and coordination deficits rarely resolve when present at the time of diagnosis. The recurrence rate for metastatic epidural SCC after successful treatment of the initial compression is 7% to 14%.42
Cranial Nerves
Cranial nerve palsies are caused by tumors that either originate near the base of the skull or metastasize there. Cancer can directly invade cranial nerves or exogenously compress them. Tumors often invade the neural foramina, which is seen in 15% to 35% of patients with nasopharyngeal carcinoma (a highly neurotrophic cancer).264 Bone metastases from lung, breast, and prostate cancers involving the base of the skull are also common sources of cranial nerve compromise.209 The incidence with which different cranial nerves are affected by cancer remains poorly quantified. One series of breast cancer patients reported a 13% incidence of cranial nerve dysfunction.90 The trigeminal and facial nerves were most frequently involved.
Clinical presentations vary depending on the cranial nerve being compressed. Evaluation should include MRI, which is the diagnostic test of choice.206 If patients have a bone-avid tumor (e.g., lung, breast, or prostate), a CT scan should be considered because bone destruction is more easily observed on CT scan.189 Positron emission tomography (PET) scanning, particularly in conjunction with CT scanning, can help to discretely localize tumor if extensive postradiation change or surgical alteration of the bony architecture has occurred. Acute management should include oral steroids, unless contraindicated, to preserve neurologic function until definitive treatment is delivered. Treatment generally involves chemotherapy and radiation.202
Spinal Roots
Malignant radiculopathies arise through direct hematogenous spread to the nerve roots or dorsal root ganglia, or more commonly by invasion from the paravertebral space. When the latter occurs, tumor can grow longitudinally in the paravertebral space and concurrently invade multiple foramina to produce a polyradiculopathy.202 Most cancer-related radiculopathies initially produce dysesthetic, aching, or burning pain in the affected dermatome, which can be associated with lancinations. Sympathetic hyperactivity or hypoactivity can be present.50 Involvement of the lower cervical or upper thoracic roots can produce Horner syndrome. In patients with a history of cancer, a new case of Horner syndrome should be attributed to malignancy until proven otherwise. Patients can complain of muscle cramps in affected myotomes.244
Nerve Plexuses
The brachial and lumbosacral plexi are commonly compressed or invaded by tumor. The frequency of neoplastic brachial plexopathy is 0.43%, and lumbosacral plexopathy 0.71%, based on retrospective case series.117,132 The most common sources of brachial plexopathy are tumors at the lung apex and regional spread of breast cancer.132 Because cancer generally grows superiorly to invade the lower brachial plexus, the inferior trunk and medial cord are most commonly involved. Occasionally head and neck neoplasms grow inferiorly to invade the upper trunk.116
Pain in the shoulder region and proximal arm occurs in 89% of patients with malignant brachial plexopathy and is the most common presenting symptom.133 The presence of pain helps to distinguish malignant from radiation-induced plexopathy. Only 18% of patients with radiation-induced plexopathy develop pain.133 Radiation plexopathies also differ in their propensity to cause progressive weakness in the C5–C6 myotomes as opposed to the lower cervical levels.133 Horner syndrome occurs in 23% of cancer patients with malignant brachial plexopathies.116 The presence of Horner syndrome suggests potential neuroforaminal encroachment and SCC. Numbness and paresthesias associated with malignant plexopathies typically are perceived in the C8 dermatome, especially digits 4 and 5.202 Loss of hand dexterity and power can be the initial motor complaint. Weakness subsequently extends proximally to involve the finger flexors, wrist extensors and flexors, and elbow extensors.202
Malignancies responsible for lumbosacral plexopathies include colorectal carcinomas; retroperitoneal sarcomas; or metastatic tumors from breast, lymphoma, uterus, cervix, bladder, melanoma, or prostate.117 When primary intrapelvic neoplasms are not responsible, the lumbosacral plexus is generally invaded from lymphatic and osseous metastases.116 Sacral plexopathies are more common than those in the lumbar region. Lumbar and sacral plexopathies can also occur concurrently.202 Lumbosacral plexopathies are bilateral in 25% of patients, particularly when the sacral plexus is more extensively involved.116,202 Incontinence and impotence strongly suggest bilateral involvement.117 Back, buttock, and/or leg pain is present in 98% of patients with malignant lumbosacral plexopathies. Among the 60% of patients who eventually develop neurologic deficits, 86% have leg weakness and 73% sensory loss.116 Positive straight leg raise is present in more than 50% of patients.117 As many as 33% of patients complain of a “hot dry foot” resulting from involvement of sympathetic components of the plexus.50
Diagnosis and Treatment
The evaluations of a suspected brachial plexopathy should include chest radiography to assess the lung apex. MRI with gadolinium is the diagnostic test of choice for evaluating the brachial and lumbosacral plexi.251 Cancerous invasion of plexi can extend along adjacent connective tissue or the epineurium of nerve trunks, without producing a discrete mass.65 For this reason, MRI findings can be erroneously interpreted as postradiation change. Electromyography can distinguish plexopathies from radiculopathies by defining the distribution of denervation. The presence of myokymia on needle examination is believed to be pathognomonic for radiation plexopathy.93
Acute treatment should include steroids for preservation of neurologic function. Radiation can effectively relieve pain but is less helpful in restoring lost function.116 Chemotherapy is commonly initiated or altered when plexus involvement heralds cancer progression; however, the success of this approach remains poorly characterized. Refractory pain requires aggressive coadministration of opioid and adjuvant analgesics, and potentially high cervical cordotomy or rhizotomy.113 Stellate ganglion blockade can relieve pain that is caused by sympathetically involvement.
Peripheral Nerves
Peripheral nerves are affected most often by cancer when extension of a bone metastasis produces a mononeuropathy.213 Rare polyneuropathy or mononeuritis multiplex resulting from myeloma, lymphoma, or leukemia has been reported.118,162 More commonly, nerves are compressed where they pass directly over an involved bone or through a bony canal.213 Common sites of nerve compression include the radial nerve at the humerus, obturator nerve at the obturator canal, ulnar nerve at the elbow and axilla, sciatic nerve in the pelvis, intercostal nerves, and peroneal nerve at the fibular head. Pain generally precedes motor and sensory loss.202
Paraneoplastic Syndromes
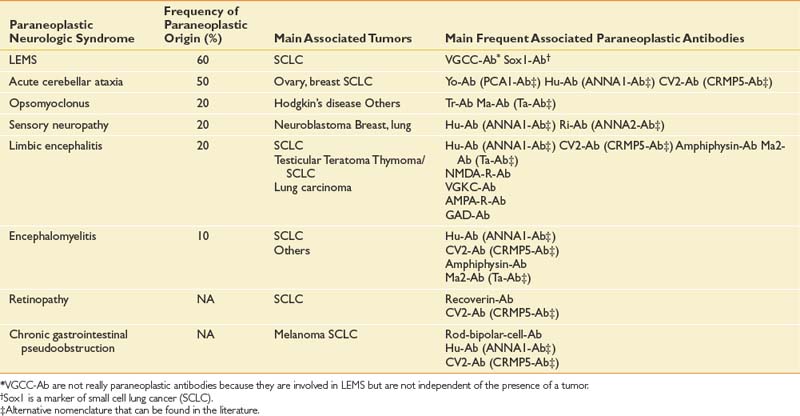
Paraneoplastic syndromes are pertinent to rehabilitation because they produce refractory neurologic deficits and severe disability.58 The incidence of paraneoplastic neurologic disorders (PNDs) is low, occurring in less than 1% of all cancer patients.106 PNDs can affect any level of the nervous system. Classic PNDs are listed in Table 57-4. These syndromes are produced when antibodies are made against tumors that express nervous system proteins. Discrete or multifocal neural degeneration produces diverse symptoms and deficits. Most PNDs are triggered during the early stages of cancer, when primary tumors and metastases might be undetectable by conventional imaging techniques. The emergence of a PND in a patient with known cancer should trigger workup for recurrent or progressive disease. PNDs are characterized by symptoms that develop and progress rapidly in days to weeks, and then stabilize. Spontaneous improvement is rare. Diagnostic workup can include serum and cerebrospinal fluid studies, brain MRI, and PET.5,47 Screening patients’ serum or cerebrospinal fluid for antineuronal antibodies known to be associated with particular cancers can direct the search for an occult malignancy. Timely diagnosis and treatment of the tumor offer the greatest chance of success in managing PNDs.11,35 PNDs do not generally respond solely to immunotherapies, including intravenous immunoglobulin, corticosteroids, and immunosuppressants. However, these can be useful adjuvant treatments.
Rehabilitation of patients with PNDs is determined by the type, distribution, and severity of the associated neurologic deficits. Potential improvement with planned antineoplastic therapy should be taken into consideration. Supportive and preventive measures to protect the integrity of the skin, affected joints, and genitourinary symptoms are critical while awaiting stabilization of neurologic deficits. Communication, respiratory, and nutritional issues should be addressed in patients with bulbar involvement.
Skin Metastases
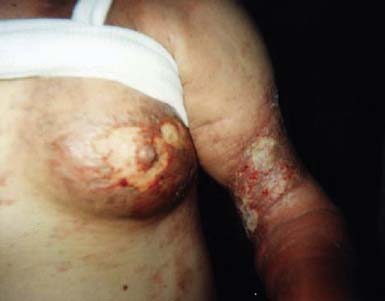
Dermal metastases occur in 5.3% of patients and are most common in breast cancer.134 Skin metastases can be a source of pain and an entry point for infectious pathogens. Because the associated wounds seldom heal, chronic wound care is necessary and becomes an integral part of patients’ rehabilitation needs. Figure 57-1 shows a breast cancer patient with dermal metastases involving the breast and proximal arm. Dermal metastases often engender or aggravate lymphedema. Use of compression is limited only by patient tolerance. Malignant wounds should be managed with nonadherent, bacteriostatic, hyperabsorbent dressings (e.g., SilvaSorb or Aquacel Ag). Associated pain must be managed aggressively to minimize adverse functional consequences. Proactive range of motion (ROM) activities can prevent the formation of contractures in joints adjacent to malignant wounds, facilitating hygiene and autonomous self-care.
Cardiopulmonary Metastases
Lung, pleural, and pericardial metastases involving the heart and lungs can produce dramatic and abrupt reductions in patients’ stamina and functional status. Virtually all cancers have the potential to spread to the lungs and pleura. At autopsy, 25% to 30% of all cancer patients have lung metastases.49 Pleural metastases occur in 12% of breast and 7% to 15% of lung cancers.135,138 Metastases to the heart and pericardium are less common, although their functional impact can be similarly devastating. A series of 4769 autopsies revealed the presence of cardiac metastases in 8.4% of cancer patients.237 Melanoma, mesothelioma, lung tumors, and renal neoplasms had the highest prevalence of cardiac spread. The clinical diagnosis of heart or lung metastases can be generally made by CT scans. PET scans and plain radiographs can also be helpful, depending on the clinical context.
Treatment of lung, pleural, pericardial, or cardiac metastases varies considerably. The type and efficacy of anticancer treatment will depend on the primary tumor, number and location of metastases, previous antineoplastic therapies, overall medical condition of the patient, and degree of associated symptomatic distress. Surgical metastectomy has the potential to definitively eliminate disease in certain patients.260 Discrete metastases that are not resectable might be amenable to radiation therapy. Patients with extrathoracic metastases are commonly treated with systemic chemotherapy.
Malignant pleural effusions should be evacuated when patients become symptomatic. The associated dyspnea often arises from other causes as well, and reducing the effusion might fail to alleviate patients’ shortness of breath if the lung is trapped because of parenchymal or pleural disease. Reaccumulation of malignant effusions can be managed through intermittent thoracentesis or pleurodesis, or placement of an indwelling pleural catheter.39 Chemical pleurodesis has an overall complete response rate of 64% when all sclerotic agents are considered.138 Talc appears the most effective, with a complete response rate of 91%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree