CHAPTER 25 Calcifying Tendinitis*
Calcifying tendinitis of the rotator cuff is a common disorder of unknown etiology in which calcium crystals deposited in a living tendon in the course of reactive calcification usually undergo over time spontaneous resorption followed by healing of the tendon. During the phase of calcium deposition, the patient either may be free of pain or suffers only a mild to moderate degree of discomfort, but the disease becomes acutely painful when the calcium is being resorbed.
HISTORICAL REVIEW
The subacromial–subdeltoid bursa was recognized as a source of painful shoulders by Duplay1 as early as 1872, and he described the condition as scapulohumeral periarthritis, later also called Duplay’s disease.2,3 Numerous other terms have since been used. With the advent of roentgenography, a more exact localization became possible. Painter4 was the first to demonstrate the radiologic appearance of the disease, but he assumed a bursal localization of calcific deposits. Stieda5 and Bergemann6 also believed that the radiologic findings were in favor of a bursal localization. However, surgical exploration soon established that the calcification was primarily in the rotator cuff tendons.7,8 In his classic textbook on the shoulder, Codman9 stated definitively: “The deposits do not arise in the bursa itself, but in the tendons beneath it.” Wrede7 gave a masterly description of the disease, including the pathologic changes in the tendon: “The cells resemble more and more chondrocytes, meanwhile the fiber arrangement of the tendon is lost.”
The intratendinous localization of calcification has been repeatedly confirmed by later authors.3,10,11 Nonetheless, such confirmation did not stop the proliferation of newer nomenclature, and terms such as peritendinitis calcarea,10 periarthropathy,12 and calcified peritendinitis13 are well known. In the English literature, calcific tendinitis or calcified tendinitis is more generally accepted. We, however, prefer calcifying tendinitis, a term that to our knowledge was first used by Plenk14 and later by De Sèze and Welfling (tendinite calcifiante).15 This term denotes the evolutionary process that is directed at spontaneous healing. It clearly sets reactive calcifications apart from degenerative calcifications that are known for their progressive deterioration. Whereas in Europe the term tendinosis is most often used,16 in North America the term tendinitis is given preference.
ANATOMY
The cuff tendons that blend with the capsule of the glenohumeral joint before insertion into bone consist of the supraspinatus in its most superior portion, the infraspinatus and teres minor posterior and posteroinferior to the supraspinatus, and the subscapularis anterior to the supraspinatus. The first three tendons insert into the greater tuberosity, whereas the subscapularis attaches to the lesser tuberosity. At the zone of the tendon where calcification takes place, we were unable to distinguish histologically between the deeper, more collagenous part of the tendon and the joint capsule, a fact already noted by Codman.9 Cells of the synovial layer were often inconspicuous.
The supraspinatus tendon is the most common site of cuff tendinopathy. It is 2 to 3 cm long and traverses the subacromial compartment that is rigidly limited by the coracoacromial arch above and the humeral head below. Codman9 pointed out that diseases in the supraspinatus tendon tend to occur in a specific area of the tendon: “about half an inch proximal to the insertion.” He called this area the critical portion, which was later renamed the critical zone by Moseley and Goldie.17 The vascularity of this area has been repeatedly investigated because hypoperfusion is believed to initiate degenerative changes that subsequently result in calcification or tear.
The rotator cuff tendons are regularly supplied by the suprascapular, anterior circumflex humeral, and posterior circumflex humeral arteries. In addition, contributions are received from the thoracoacromial, suprahumeral, and subscapular arteries in descending order of frequency.18 The vascularity of the cuff tendons, especially that of the supraspinatus, has been studied in many cadaver shoulders by microangiography simultaneously with histology. Moseley and Goldie17 found that the supraspinatus was well supplied by a network of vessels coming from both the muscular and the osseous ends of the tendon and that they anastomosed in the area of the critical zone. They stated that there is “no evidence that the critical zone is much less vascularized than any other part of the tendinous cuff.” On the other hand, microangiographic studies by Rothman and Parke19 showed that the critical zone was markedly undervascularized, and their histologic examinations corroborated this view. Although the supraspinatus tendon was most commonly involved, the infraspinatus and subscapularis tendons showed zones of hypovascularity as well. Brooks and associates20 performed a quantitative histologic study of the cuff and concluded that the supraspinatus and infraspinatus are equally hypovascular in their distal 15 mm and that the diameter of vessels decreases toward the bony insertion.
Rathbun and Macnab21 documented in their cadaver study a zone of avascularity in the supraspinatus tendon near its bony insertion. This avascularity depended on the position of the arm. They showed that when barium sulfate (Micropaque) was injected into the vessels with the arm of the cadaver in the position of adduction, the critical zone did not fill. The nonfilling area sometimes extended up to the point of insertion. If the vessels of the contralateral shoulder of the same cadaver were injected with the arm in abduction, the vessels filled completely throughout the tendon. Therefore, the authors postulated that the zone of avascularity was a wring-out effect resulting from pressure of the head of the humerus on the tendon. In another study, Tillmann22 documented that the zone of insertion of the supraspinatus is avascular because of pressure or compression.
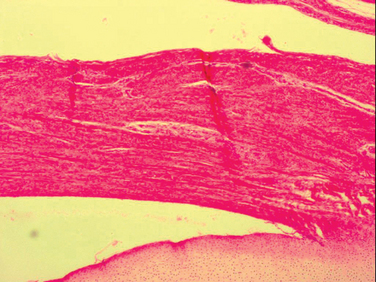
Our histologic findings have been similar to those of some of these authors.23 There is hardly an area in the supraspinatus tendon that is conspicuously devoid of vascular channels. However, we have observed that vascular channels, especially the larger ones, are abundant in the loose connective tissue underneath the subacromial bursa but are relatively scarce in the dense collagenous portion of the tendon close to the joint. Interestingly, this pattern of vascularity is already evident in the supraspinatus tendon of the fetus, even before the fascicular arrangement of the tendon fibers has occurred (Fig. 25-1).
Our histologic studies of cadaver tendons were supplemented by microangiographic studies that consistently showed an area of underfilling at the deeper, articular part of the tendon near its insertion, regardless of the position of the arm.23
INCIDENCE
Reports on the overall incidence of tendon calcification vary tremendously. This variation depends not only on the clinical material used but also on the radiographic technique. Bosworth examined both shoulders of 6061 office workers and found an incidence of calcification of 2.7%.24,25 Welfling and colleagues26 radiographed 200 shoulders of persons without any complaints and found calcifications in 15 (7.5%). Rüttimann27 radiographed 100 persons without symptoms and found a 20% incidence of calcification.
The incidence of calcification in 925 painful shoulders reported by Welfling and collaborators26 was 6.8%. When broken down by age group, patients aged between 31 and 40 years had a 19.5% incidence of calcification. Evidently, the peak at this age did not correspond with the peak seen in patients with rotator cuff tears. They therefore concluded that both diseases represent different entities. Friedman radiographed shoulders of 228 patients with a painful rotator cuff and found calcific deposits in 75.28 Fifty-four of the 75 patients with calcification were between 30 and 49 years of age. Bosworth estimated that 35% to 45% of patients with deposits will eventually become symptomatic, pointing again to the fact that the deposition of calcific material is devoid of symptoms.24,25
Plenk14 found that 82% of the calcifications were located in the supraspinatus tendon. Bosworth24,25 found 90% in the supraspinatus and infraspinatus. DePalma and Kruper29 reported an incidence of 74% when assessing the supraspinatus alone, whereas the incidence of simultaneous calcific deposits in the supraspinatus and other short rotators was 90%. In Bosworth’s series,24,25 calcifications in the supraspinatus occurred in 51%, in the infraspinatus in 44.5%, in the teres minor in 23.3%, and in the subscapularis in 3%. Obviously, deposits were sometimes seen in more than one tendon. In Hsu and collaborators’ study of 82 patients, 58 (70.7%) had a deposit in the supraspinatus, 22 (26.8%) had a deposit in the infraspinatus, and 2 had a deposit in the teres minor.30
In general, authors agree that women are affected more often than men. Bosworth24,25 reported an incidence of 76.7% in women; DePalma and Kruper29 reported an incidence of 60.3%; Welfling and collaborators26 reported an incidence of 62%; Lippmann31 as well as Hartig and Huth32 reported 64%; and in our series of 127 patients, the incidence was 57%. A higher incidence in men (56%) was reported by Friedman.28 An even higher incidence was seen by Hsu and coworkers.30 Of 82 patients, 61 men suffered from calcifying tendinitis (74%).
The age distribution varies slightly among authors. Welfling and collaborators26 reported the highest incidence in persons between 31 and 40 years of age, whereas DePalma and Kruper29 found calcifying tendinitis in 36% of patients in the 40- to 50-year-old group. In our series, 53 patients (42%) were in the 40- to 49-year-old group. Welfling and coworkers26 state that in their group of 925 patients, no calcification was seen in patients older than 71 years, and McLaughlin33 reported that no calcification was detected in about 1000 older cadavers in an anatomy laboratory. It seems that men peak slightly later than women do in regard to the incidence of this disease. Lippmann31 had similar results: The average age of women was 47 years and that of men was 51 years. Hsu and associates30 reported that 56 of 82 patients were older than 60 years. They concluded that sex and age of patients with calcifying tendinitis are different in Asians. Nutton and Stothard34 reported the presence of calcifying tendinitis in a 3-year-old child.
Occupation seems to play a role in calcifying tendinitis. In DePalma and Kruper’s group,29 41% were housewives and 27% were professionals, executives, and salespersons. In our series, 43% were housewives, and 44% were people who held clerical jobs.
The right shoulder is usually affected more often than the left, amounting to 64% in Hartig and Huth’s study,32 57% in DePalma and Kruper’s series,29 and 51% in our series. Bilateral involvement was found in 24.3% by Welfling and coworkers.26 In DePalma and Kruper’s series,29 the incidence was 13%. Of our patients, 17% came back with calcification of the opposite shoulder; this incidence increases with increasing length of the follow-up period.
All authors agree that calcifying tendinitis is not related to any generalized disease process, and Welfling and colleagues26 rightly conclude that tendon calcifi-cation constitutes a disease entity on its own. Neither McLaughlin33 nor Rüttimann27 could find a correlation between tendon tear and calcific tendinitis. Partial tears in patients with calcifying tendinitis occur mostly on the bursal side when the deposit ruptures into the bursa. Ruptures into the glenohumeral joint are said to occur extremely seldom.35 Patte and Goutallier36 observed two cases. Hsu and collaborators30 found arthrographic evidence of a complete rotator cuff tearing in 20.7% (17 patients) and a partial joint-side tear in 6 patients (7.3%) among the 82 patients with calcific tendinitis. We did not record a single instance of a complete cuff tear. Morimoto and colleagues37 observed calcification of the coracoacromial ligament in one patient.
CLASSIFICATION
Several classifications of calcifying tendinitis have been proposed. DePalma29 classified calcifying tendinitis into two types: type I, fluffy and amorphous; type II, defined and homogenous. Type I calcifications are most commonly seen in patients with acute symptoms, and type II calcifications are most commonly observed in patients with chronic symptoms. However, Maier38 determined that the intraobserver reproducibility and interobserver reliability of the DePalma classification system was only satisfactory with kappa values of 0.487 and 0.234, respectively. The French Arthroscopic Society39 defined four types of deposits:
Gärtner40 proposed a classification that was found to correlate with the frequency of resorption after needling. Type I deposits were sharply outlined and densely structured. Type III deposits had a cloudy outline and were transparent in structure. Type II deposits had were features of both. The author noted that resorption following needling occurred in 33%, 71%, and 85% for types I, II, and III, respectively.
The radiographic appearance of the calcifications must be interpreted within the context of the overall clinical picture. Thus the acuity or chronicity of the symptoms and the cyclical nature of the disease must also be taken into account. It is, however, important to remember that radiologically visible calcifications in the cuff tendons can occur with diseases other than calcifying tendinitis. Dystrophic calcifications can be seen around the torn edges of the tendon after a complete tear.41 Dystrophic calcification associated with a tear has a poor prognosis and indicates progressive degenerative changes; it is not comparable to the spontaneous healing of the tendon in calcifying tendinitis. Moreover, dystrophic calcifications, unlike reactive calcifications, do not occur in the mid tendon but arise much closer to the bony insertion.
PATHOLOGY
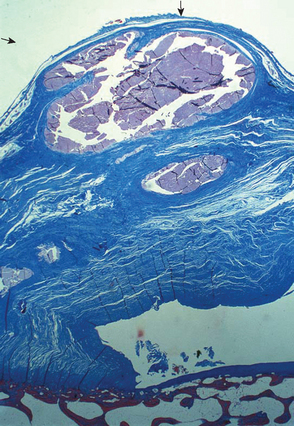
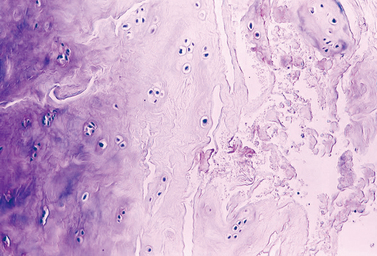
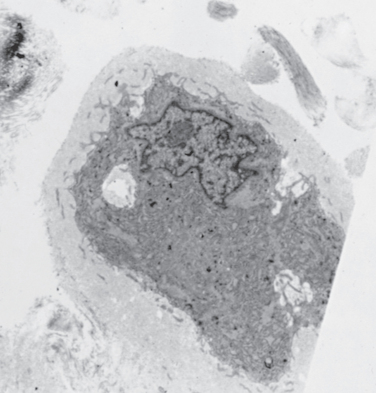
Under low magnification, the calcific deposits appear multifocal, separated by fibrocollagenous septa or fibrocartilage (Fig. 25-2). Higher magnification reveals easily distinguishable chondrocytes, described by Archer and collaborators42 as chondrocyte-like cells within a matrix showing varying degrees of metachromasia (Fig. 25-3). The calcium deposits may be loosely granular or appear in clumps (Fig. 25-4). The appearance of chondrocytes within the tendon substance near calcifications was noted by Wrede7 in 1912, by Harbin43 in 1929, by Sandström and Wahlgren10 in 1937, by Howorth44 in 1945, and by Pedersen and Key in 1951.45 The ultrastructure of these chondrocytes shows that the cells often have a fair amount of cytoplasm containing a well-developed endoplasmic reticulum, a moderate number of mitochondria, one or more vacuoles, and numerous cell processes (Fig. 25-5). The margin of the nucleus is indented. The cells are surrounded by a distinct band of pericellular matrix with or without an intervening lacuna. With the transmission electron microscope, aggregates of rounded structures containing crystalline material are found in a matrix of amorphous debris or irregularly fragmented collagen fibers. When examined by scanning electron microscopy, calcific deposits appear as rocky bulks engulfed in mortar.46 The irregularly rectangular crystals are sometimes found within membrane-bound structures resembling matrix vesicles, also called calcifying globules.47-50 Infrequently, crystalline densities seem to be embedded between collagen fibers.
The fibrocartilaginous areas are generally avascular. The intercellular substance is metachromatic, and glycosaminoglycan-rich pericellular halos are prominent around rounded cells.42 Surprisingly, monoclonal collagen staining by Archer and colleagues42 did not reveal the presence of type II collagen. In our studies using type II collagen monoclonal antibodies, we occasionally documented its presence. The difference in outcome may be due to differences in tissue preparation, source of monoclonal antibodies, and staining technique. In contrast to the fibrocartilage, the fibrocollagenous tissue abutting the calcification can appear compressed, with formation of a pseudocapsule around the deposits. The neighboring tendon fibers can show thinning and fibrillation. Examination by chemical methods, x-ray diffraction, and infrared spectrometry, as well as thermogravimetry, has shown that the crystals are carbonated apatite.51 However, high-resolution transmission electron microscopy reveals that the crystals are much larger than the classic apatite crystals and have a different configuration.52
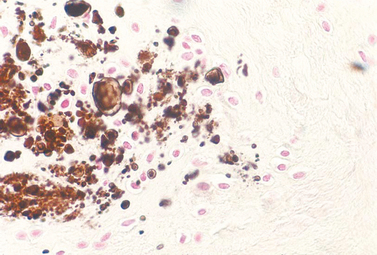
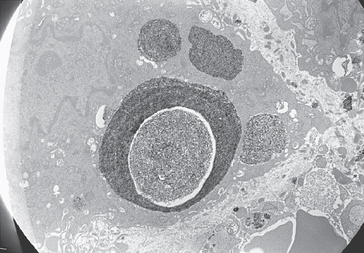
In 1915, Moschkowitz53 identified deposits within the tendon but stated that they failed to evoke a cellular reaction. A few years before, however, Wrede7 had already written that although inflammation or vessels were notably absent around some deposits, “young mesenchymal cells, epithelioid cells, leukocytes, a certain number of lymphocytes and occasionally giant cells” were present at other sites of calcification. The presence of these cells is compatible with resorptive activity at that stage. Indeed, the marked cellular reaction around calcific deposits—the “calcium granuloma”—was considered by Pedersen and Key45 to be the characteristic lesion of calcifying tendinitis. The granulomatous appearance is imparted by the presence of multinucleated giant cells and macrophages (Fig. 25-6). Archer and coauthors42 interpreted the presence of the latter two cell types as a resorption phenomenon. Capillary or thin-walled vascular channels around the deposits often accompany the cellular reaction. The margin and, more seldom, the interior of the deposits are infiltrated by macrophages and a few leukocytes, including polymorphonuclear cells and fibroblasts. At the end of the resorptive phase, phagocytosed substance within macrophages or multinucleated giant cells can often be discerned. The ultrastructure of these cells shows electron-dense crystalline particles in cytoplasmic vacuoles, but the crystals are slightly different in appearance from those in the extracellular deposits.54 Some of the intracellular accumulations have a rounded aspect and are known as microspheroliths or psammomas (Fig. 25-7).
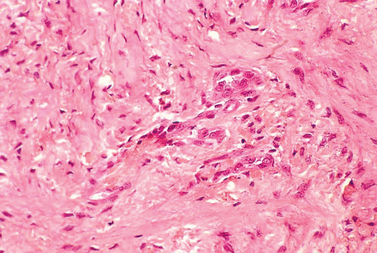
Small areas representing the process of repair can be found in the general vicinity of calcification, and these areas show considerable variation in appearance. Granulation tissue with young fibroblasts and newly formed capillaries contrasts with well-formed scars with vascular channels and maturing fibroblasts that are in the process of alignment along the long axis of the tendon fibers (Fig. 25-8). Using monoclonal antibodies against collagen type III, we were able to confirm collagen neoformation, most pronounced around vascular channels (Fig. 25-9).
Calcific deposits in the wall of the subacromial bursa also tend to be multifocal. A cellular reaction is seldom seen around the bursal deposits.55
PATHOGENESIS
Other Opinions
Codman9 proposed that degeneration of the tendon fibers precedes calcification. The fibers become necrotic, and dystrophic calcification follows. Degeneration of fibers of the rotator cuff tendons because of a wear-and-tear effect and aging has been postulated or demonstrated by many investigators. Obviously, these two causes are interrelated. It is reasonable to assume that the rotator cuff tendons suffer a wear-and-tear effect because the glenohumeral joint is not only a universal joint but probably also the most used joint in the body. Studies performed in Sweden seem to indicate that stress and strain induced by work involving the arm can lead to supraspinatus tendinitis.56 However, there are no indications that calcifying tendinitis would develop in time, even in a worker engaged in heavy manual labor. Olsson57 has shown that the cuff tendons from the dominant arm show no more evidence of degeneration than those from the contralateral arm.
Aging is considered to be the foremost cause of degeneration in cuff tendons. Brewer58 believes that with aging comes a general diminution in the vascularity of the supraspinatus tendon along with fiber changes. The well-delineated bundles of collagen or the fascicles that constitute the distinctive architecture of the tendon show the most conspicuous age-related changes, beginning at the end of the fourth or fifth decade.57 The majority of the fascicles undergo thinning and fibrillation, which is defined as a degenerative process characterized by splitting and fraying of the fibers. The thinned fascicles show irregular cellular arrangement, and the fragmented fibers are often hypocellular. The intervening connective tissue that carries the blood vessels between the fascicles can appear to be increased when contrasted with the volume of the fascicles. In our experience, it is difficult to ascertain the numerical decrease in vessels, but more vessels with thicker walls are consistently found in the cuff tendons of aged persons.
Because calcifying tendinitis seldom affects persons before their fourth decade, it may be argued that primary degeneration of tendon fibers is responsible for the subsequent deposition of calcium. Following Codman’s suggestion of the degenerative nature of calcifying tendinitis,9 subsequent investigators have found ample histologic evidence for the sequence of degeneration, necrosis, and calcification.59–61 According to McLaughlin,33 the earliest lesion is focal hyalinization of fibers that eventually become fibrillated and get detached from the surrounding normal tendon. Continued motion of the tendon grinds the detached, curled-up fibers into a wen-like substance consisting of necrotic debris on which calcification occurs. This sequence of events was demonstrated experimentally by Macnab62 in the course of investigations on the effects of interruption of the vascular supply to the Achilles tendon of rabbits. Pedersen and Key,45 who are also in favor of the concept of degenerative calcification, state that “calcium is deposited in the necrotic collagenic tissue.”
Mohr and Bilger63 believe that the process of calcification starts with necrosis of tenocytes along with a concomitant intracellular accumulation of calcium, often in form of microspheroliths, also known as psammomas. Contrary to Mohr and Bilger,63 we never observed psammomas during the early phases of formation, but we did observe them regularly during the phase of resorption. Our electron-microscopic examinations leave no doubt that the electron-dense material is situated intracellularlyand not extracellularly, as reported by other authors. The biochemical aspects of soft tissue calcifications have been published by Seifert.64
Authors’ Opinion
The aforementioned investigators failed to differentiate between two fundamentally distinct pathogenetic processes: the reactive, self-healing process as present in calcifying tendinitis, and the degenerative, progressive process as found in dystrophic calcifications. Lippmann31 observed in instances of calcifying tendinitis that “the early deposit, located in a totally avascular bed, provokes no tissue reaction.” He contrasted this observation with “the mechanism of absorption that primarily is dependent upon vascularity and inflammation.” Moreover, Jones65 deplored the fact that “proper assessment of the natural repair process in acute cases has not been made.”
We would like to add that neither the self-healing nature of calcifying tendinitis nor the various aspects of its pathology and clinical evolution are characteristic of a degenerative pathology. We believe that the process of calcification in calcifying tendinitis is actively mediated by cells in a viable environment.54,66–73 On the other hand, no spontaneous disappearance of degenerative calcifications has ever been described; to the contrary, they progress with advancing age.
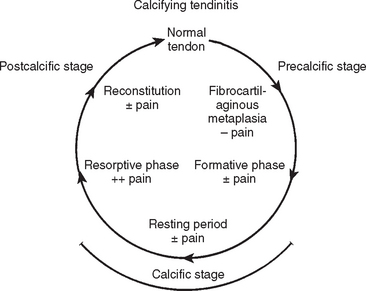
Calcifying tendinitis goes through three distinct stages (Fig. 25-10): a precalcific stage (metaplasia of matrix), calcific stage (calcification of matrix and resorption of calcific deposits), and a postcalcific stage (reconstitution of matrix).
Precalcific Stage
In the precalcific stage, the site of predilection for calcification undergoes fibrocartilaginous transformation. This metaplasia of tenocytes into chondrocytes is accompanied by metachromasia of the matrix that indicates the elaboration of proteoglycan.
Calcific Stage
The calcific stage is subdivided into the formative phase and the resorptive phase.
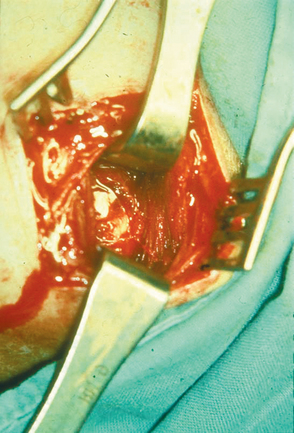
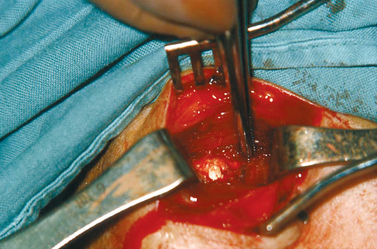
In the calcific stage, calcium crystals are deposited around chondrocytes primarily in matrix vesicles that coalesce to form large areas of deposits.54 For convenience of description, we have used the term formative phase to describe the initial period of the calcific stage.70 If the patient undergoes surgery at this stage, the deposit appears chalk-like (Fig. 25-11) and has to be scooped out for removal. During this phase, the area of fibrocartilage with the foci of calcification is generally devoid of vascular channels. Septa of fibrocartilage separating the foci of calcification stain metachromatic. They do not consistently stain positive for collagen type II, which is known to be a component of fibrocartilage. These fibrocartilaginous septa are gradually eroded by the enlarging deposits.
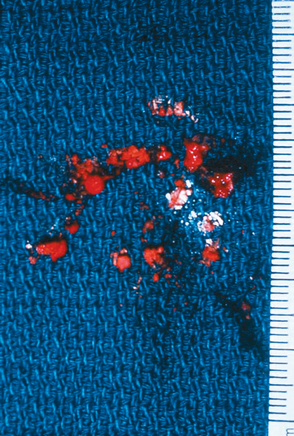
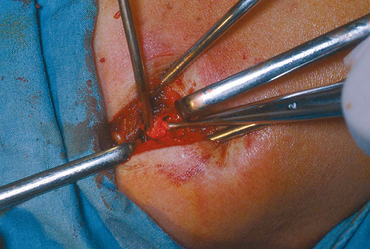
After a variable period of inactivity of the disease process (“resting period” in the schema), the spontaneous resorption of calcium is heralded by the appearance of thin-walled vascular channels at the periphery of the deposit. Soon thereafter, the deposit is surrounded bymacrophages and multinucleated giant cells that phagocytose and remove the calcium. This step is the last one in the calcific stage, which we have called the resorptive phase. If an operation is performed at this stage, the calcific deposit is a thick, white, cream-like or toothpaste-like material and spurts out when the area of the deposit is incised (Fig. 25-12).
Postcalcific Stage
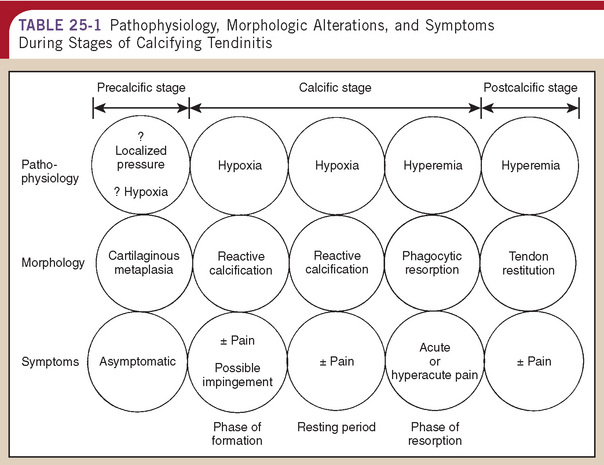
Simultaneously with the resorption of calcium, granulation tissue containing young fibroblasts and new vascular channels begins to remodel the space occupied by calcium deposit. These sites stain positive for collagen type III. As the scar matures, fibroblasts and collagen eventually align along the longitudinal axis of the tendon. During this remodeling process, type III collagen is replaced by type I. We have called this stage of tendon reconstitution postcalcific. Table 25-1 outlines the correlation between pathogenesis, morphologic findings, and symptoms during the various stages of calcifying tendinitis.
TABLE 25-1 Pathophysiology, Morphologic Alterations, and Symptoms During Stages of Calcifying Tendinitis
Although the pathogenesis of the calcifying process can be reasonably constructed from morphologic studies, it is difficult to determine what triggers the fibrocartilaginous transformation in the first place. Codman9 suggested tissue hypoxia as the primary etiologic factor. This hypothesis still remains attractive because of the peculiarity of the tendon vasculature and shoulder mechanics. We have found an increased frequency of human leukocyte antigen (HLA)-A1 in patients with calcifying tendinitis, thus indicating that these patients may be genetically susceptible to the condition.74 Factors that trigger the onset of resorption also remain unknown.
Our phase of formation seems identical to Lippmann’s early phase of increment,31 whereas his late phase of increment is analogous to our phase of resorption.
CLINICAL FINDINGS
Clinical keys identifying calcifying tendinitis are as follows: pain, most pronounced during the resorptive phase; reduced range of motion, mostly secondary to pain; radiologic evidence of intratendinous calcification; and positive sonographic findings. Pain is the cardinal symptom of calcifying tendinitis.75–79
We would like to strongly emphasize that an understanding of the pathogenetic mechanism of calcifying tendinitis is essential for clinical evaluation and management of this disease entity. There is a tendency to assume that calcifying tendinitis, as in most diseases, begins with acute symptoms and progresses to a chronic state. Others maintain that we are dealing with two separate disease entities. Our understanding of the disease is entirely to the contrary. We believe that the initial stage of formation of the deposit, which lacks a vascular and cellular reaction, is likely to cause few symptoms or just nondebilitating discomfort because the intratendinous tissue tension is hardly raised by the deposit. Thus, the disease generally begins with chronic symptoms, if any at all. Larger deposits can lead to impingement against the coracoacromial ligament, a fact already observed by Baer80 in 1907 during surgery. During the later phase of calcium resorption, on the other hand, exudation of cells along with vascular proliferation must enlarge the tissue space considerably and thus raises the intratendinous pressure, a process that causes acute pain. The pain is probably further exacerbated as the increased volume of the tendon impinges on the unyielding structures that limit the subacromial compartment.
In fact, the subclinical nature of the formative phase of calcification has been recognized by many authors. Codman9 stated that “the usual history is not acute pain at the beginning.” Wilson81 noted that many patients might know about a calcium deposit in one or both shoulders for months or years before an acute attack. Lippmann31 stressed the well-known fact that early deposits are usually symptomless and that acute pain signals “the onset of ‘break-up’ of the deposit.” Pinals and Short82 wrote, “calcium deposition precedes rather than follows the development of an acute attack of calcific periarthritis and the attack is accompanied by disintegration and gradual disappearance of the deposit.” Similarly, Gschwend and associates35 believed that the calcification is often symptomless at the beginning, whereas its disappearance is associated with pain.
It is therefore evident that we are not dealing with two unrelated disease processes, an acute and a chronic calcific tendinitis, but a disease cycle. Lippmann31 described a phase of increment followed by a short, self-limited phase of disruption. Each phase has its characteristics. During the phase of increment, the symptoms were described as being mild, the consistency of the deposit was said to be hard and chalky, and no inflammation was noted. During the phase of disruption, the pain was severe, the consistency allowed tapping, and radiographs showed a fluffy deposit. Lippmann31 concluded that failure to identify the phase of the cycle resulted in crediting “useless therapeutic measures with magical healing power and, on the other hand, led to the performance of needless surgical procedures.”
Clinical findings depend on the acuteness of the symptoms. Simon83 believed that a definite relationship exists between the intensity of symptoms and their duration. The symptoms can last up to 2 weeks when they are acute, 3 to 8 weeks when they are subacute, and 3 months or more when they are chronic. Pendergrass and Hodes84 observed that the acute symptoms subside in 1 to 2 weeks, even in the absence of treatment. It is also known that symptoms can change rapidly.
During the subacute and chronic phases, patients complain of pain or tenderness. They are usually able to localize the point of maximal tenderness. Radiation of pain is the rule, the insertion of the deltoid being the most frequent site of pain referral. Referred pain was seen in 42.5% of the patients of De Sèze and Welfling.15 Radiation of pain occurred more often in the arm than toward the neck. Wrede7 and many authors after him found that clinical symptoms are often absent. Usually, range of motion is decreased by pain, patients cannot sleep onthe affected shoulder, and they often complain of an increase in pain during the night. A painful arc of motion between 70 and 110 degrees has been described by Kessel and Watson,85 but these authors were unable to classify these patients into any of their three types of the painful arc syndrome (posterior, anterior, and superior). In 97 patients with this syndrome, they found 12 cases of calcification. Patients often have the sensation of catching when going through the arc of motion. This sensation is most probably due to localized impingement, which in turn leads to loss of scapulohumeral rhythm. Impingement between the calcium deposit and the coracoacromial ligament during abduction had already been noted by Baer80 and by Wrede.7 A further sign of the long-standing symptoms of calcifying tendinitis is atrophy of both spinatus muscles. Although some authors have reported the presence of swelling and redness on clinical examination, we were never able to find these signs.
During the acute phase, the pain is so intense and excruciating that patients refuse to move their shoulders. De Sèze and Welfling15 believe that this severe pain leads to locking. Any attempt at mobilization of the glenohumeral joint is resisted by the patient. Patients hold their arms close to their bodies in internal rotation. Although we have described involvement of the bursa in painful shoulder syndromes,54 its involvement in various phases of calcifying tendinitis has not been documented in detail.
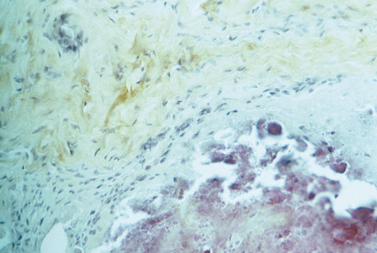
During the formative phase, the subacromial bursa is not the site of widespread reaction. Only in the presence of impingement86 is a zone of hyperemia observed around the calcium deposit (Fig. 25-13). Carnett87 noted that bursitis is a minor and infrequent feature.
During the resorptive phase, bursitis is said to be a source of pain. However, during surgery, the bursal reaction is minimal and often limited to localized hyperemia (Fig. 25-14). This reaction is not usually severe enough to cause bursal thickening. In fact, the calcific deposit often shines through the deep or visceral layer of the bursa. Litchman and colleagues88 also noted the absence of bursal inflammation. Many authors state that rupture of the deposit into the bursa causes a crystalline type of bursitis and, consequently, pain. We would like to emphasize again that only during the resorptive phase does the consistency of the deposit allow rupture into the bursa. DePalma and Kruper29 noted that deposits rupturing into the bursa are encountered in acute cases. De Sèze and Welfling15 monitored 12 patients with rupture of the deposit into the bursa. Only 8 patients had symptoms. In our histologic specimens, we could observe synovial cells resorbing calcium. No inflammatory reaction—in particular, no leukocytes or lymphocytes—accompanied this resorptive process. It therefore seems probable that the edema and proliferation of cells and vessels cause an increase in intratendinous pressure that evokes pain rather than a localized bursal reaction. This impression seems to be confirmed by intraoperative observations during the acute phase. Key89 reported: “In some hyperacute cases of short duration, the calcific material is thin or milk-like in consistency and may be under such pressure that when the surface of the tendon is incised, the contents of the deposit may spurt out into the air.” An identical observation has been made by Friedman.28
In 41 operated patients, we correlated symptoms with radiologic findings and with consistency of the deposit.66 Of 31 patients with chronic symptoms, 24 had radiographic signs compatible with formation and 29 had chalk-like granular calcium deposits. Of 10 patients withacute symptoms, 8 had radiographic signs typical of resorption and 9 exhibited a toothpaste-like consistency of their deposit.
Should calcifying tendinitis be considered a systemic disease as Pinals and Short82 have speculated? There is no good evidence despite the high incidence of calcifications occurring in other sites. With the exception of an increased incidence of HLA-A1 in patients with calcifying tendinitis, all other laboratory test results are normal.74 An associated illness was never reported,88 and Gschwend and associates35 were unable to prove an association with diabetes or gout, although this association had been repeatedly suspected by various authors but never documented.
A relationship to occupation must be suspected, given the high incidence of clerical workers observed by us and other investigators. Litchman and colleagues,88 on the other hand, stated that no relationship to occupation could be found.
A possible correlation between stiff and painful shoulders and calcifying tendinitis has been suspected for approximately 90 years.90,91 We could observe only a single case of this syndrome. Lundberg92 reported 24 patients with calcification among 232 with a frozen shoulder. This finding does not point toward a strong correlation between calcifying tendinitis and frozen shoulder.
RADIOLOGY
The calcium deposits in calcifying tendinitis are localized inside a tendon. They are not usually in continuity with bone, nor do they extend into bone. DePalma and Kruper29 observed extensions into bone in 8 of 136 patients. The only other occurrence has been reported by Toriyama and associates.93 Calcium deposits close to bone must be clearly distinguished from the stippled calcifications seen at the tendon insertion in patients with dystrophic calcification.
Initial radiographs should include anteroposterior films in neutral rotation as well as internal and external rotation. Deposits in the supraspinatus are readily visible on films in neutral rotation, whereas deposits in the infraspinatus and teres minor are best seen in internal rotation. Calcifications in the subscapularis occur only in rare cases, and a radiograph in external rotation shows them well. Scapular views can help determine whether a calcification is causing impingement. Ruptures into the bursa appear as a crescent-like shadow overlying the actual calcification and extending over the greater tuberosity, with the extent of the bursa outlined well (Fig. 25-15).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree