Calcific Tendinitis
Brian R. Wolf
Jonathan A. Donigan
Calcific tendinitis (also referred to as calcifying tendinitis and calcific tendinopathy) of the shoulder is a common disorder of unknown etiology. It is characterized by the multifocal accumulation of basic calcium phosphate crystals within the rotator cuff tendons. The deposition of calcium itself may cause mild-to-moderate chronic discomfort in some patients, but during the resorption of these calcifications many patients have significant acute pain. This acute and chronic pain can lead to restriction of range of motion and function. Various treatment modalities have been found beneficial in the management of this disorder, and many patients do well with conservative measures alone.
INCIDENCE AND EPIDEMIOLOGY
The reported incidence of tendon calcification varies, with 2.7% to 20% of asymptomatic shoulders found to have radiographic calcifications. It has been estimated that 35% to 45% of patients with deposits eventually become symptomatic. Fifty-one to ninety percent of calcifications have been reported to be located in the supraspinatus tendon.
It is important to note, however, that not all calcifications in the rotator cuff, even in a symptomatic shoulder, are due to calcific tendinitis. Dystrophic calcifications can be seen around the torn edges of a complete tear and portend a different prognosis than calcific tendinitis. Significant calcifications can also be seen in patients with rotator cuff arthropathy.
Women are more commonly affected than men, with studies finding that 57% to 76% of the calcific tendinitis occurs in female patients. The age distribution with the highest incidence has been reported in various studies to be in 31-to 50-year-olds, and there have been some reports of ethnic variation with an increased average age in Asians. Calcifications are uncommon in patients over 70 years old, and calcifications have been reported in a 3-year-old child. The right side is more commonly affected than the left, and bilateral involvement was present in 24% in one report.
There is good agreement that calcific tendinitis is not part of a generalized disease process. It is not believed that there is a relationship between trauma and calcific tendinitis, nor is there evidence of a correlation between tendon tears and calcific tendinitis. An association with diabetes and gout has been suspected, but no significant evidence of such has been documented. One study showed an association between calcific tendinitis and endocrine disorders; however, 65% of the patients with calcific tendinitis carried a diagnosis of a specific endocrine disorder (16). In that study, those with endocrine disorders had a higher rate of failure of conservative management leading to surgical intervention (47% underwent surgical intervention vs. 23% in patients without endocrine disorders).
PATHOLOGY AND PATHOGENESIS
In most cases, the multifocal calcifications are located 1 to 2 cm from the insertion of the supraspinatus tendon on the greater tuberosity (in the “critical zone”). Gartner and Heyer (1) analyzed calcific deposits and found that they consist of hydroxyapatite crystals (Ca10[PO4]6(OH)2) with a varying amount of H2O, CO3, and PO4 (1). The macroscopic appearance changed from a granular conglomerate during the chronic or formative phase to a milky emulsion in the acute or resorptive phase. The etiology of calcific tendinitis is still a matter of controversy. Degenerative calcification and reactive calcification are the two fundamentally different processes proposed as the cause of formation of calcium deposits in rotator cuff tendons.
Codman (2) believed that degeneration of the tendon fibers precedes calcification, with necrosis of fibers followed by dystrophic calcification. Degeneration occurs through “wear and tear” with aging. This wear and tear is accentuated by a diminution in the vascularity of the tendons with age. Fascicles become thin and fibrillated. Tendon fibers split and fray and are hypocellular. These changes begin at the end of the fourth or fifth decade. Since Codman’s description of degenerative calcification,
there has been some evidence supporting this sequence of degeneration, necrosis, and calcification.
there has been some evidence supporting this sequence of degeneration, necrosis, and calcification.
However, as Uhthoff et al. (3) argue this degenerative process does not accurately describe the clinical and morphologic aspects of calcific tendinitis. A purely degenerative etiology does not explain the peak of calcific tendinitis in the fifth decade when degeneration is known to increase in incidence as aging continues. In addition, a degenerative process would not include the self-healing that is seen in calcific tendinitis. Thus, they concluded that there is a separate entity appropriately called degenerative calcification, and another process of reactive calcification, which cause calcific tendinitis.
Reactive calcification occurs in a viable environment and is a cell-mediated process that is felt to occur in three distinct stages: precalcific, calcific, and postcalcific.
Precalcific stage: During this first stage, a portion of the tendon undergoes fibrocartilaginous transformation with metaplasia of tenocytes into chondrocytes. There is a concomitant elaboration of proteoglycan. Histologic evaluation reveals areas of fibrocartilaginous metaplasia that are generally avascular.
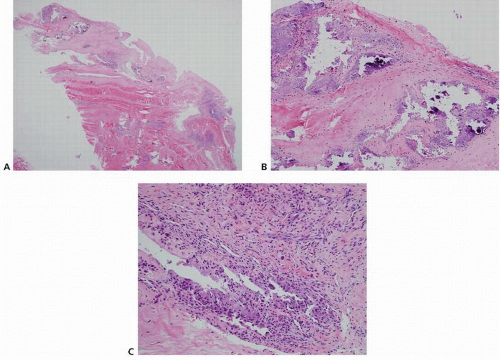
Calcific stage: The calcific stage is broken down further into formative, resting, and resorptive phases. During the formative phase, calcium crystals are deposited in matrix vesicles that coalesce to form large deposits. The enlarging deposits erode the fibrocartilaginous septa. Histologic examination shows this deposition of calcium crystals in the fibrocartilaginous matrix. The resting phase describes the period of fibrocollagenous tissue bordering the foci of calcification, indicating that deposition has ceased. The length of this period is variable. During the resorptive phase, spontaneous resorption of the calcium deposits occurs. Thin walled vascular channels appear at the periphery of the deposit. Macrophages and giant cells phagocytose and remove the calcium. Histologic examination shows this cell-mediated resorption of the calcific deposit (Fig. 25.1).
Postcalcific stage: As the calcifications are resorbed, granulation tissue with fibroblasts and vascular channels remodels the space that was occupied by the deposits. These scars remodel with fibroblasts and collagen aligned along the longitudinal axis of the tendon and type I collagen replaces type III collagen. Of note, not all foci of calcification are in the same phase in a given patient at a single point in time, but in general, one phase predominates (3).
In spite of the pathogenesis being thus described, the triggering event of the precalcific stage is unknown. It is thought by many to be related to tissue hypoxia. There is some evidence that there is a genetic component, with an increased incidence of HLA-A1 identified in patients with calcific tendinitis although other studies dispute this.
CLINICAL EVALUATION
Pertinent History
Patients with calcific tendinitis will complain of pain and a loss of range of motion. The most significant pain generally occurs during the resorptive phase, with some milder symptoms present in some patients during the formative and the postcalcific phases. The pain is believed to be caused during the resorptive phase by raised intratendinous pressure from exudation of cells and vascular proliferation. Patients are usually able to localize the pain and the point of maximal tenderness although there is often a radiation of pain with referral to the deltoid. Patients often have difficulty sleeping on the shoulder, and many complain of an increase in the pain at night. Patients often indicate that they sense catching in the shoulder as they go through an arc of motion.
Physical Exam
In cases of long-standing symptoms, patients may show atrophy of the supraspinatus or infraspinatus. Some authors have reported swelling and redness, but this has not been found by all reporters. Patients will exhibit significant tenderness in the area of the affected tendon. As mentioned above, patients usually show a loss of range of motion and may have a sensation of catching. In severe cases in the acute phase, patients may refuse to move their arms at all, and they may insist on maintaining their arms in internal rotation against their bodies. Impingement signs are frequently present although bursitis is a minor and infrequent feature based on surgical findings.
Imaging
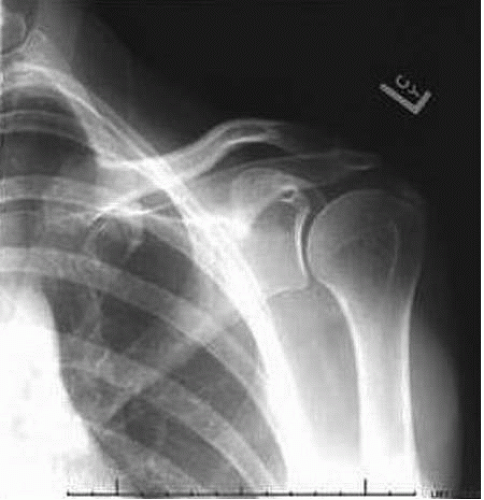
Plain radiographs will demonstrate calcifications beginning in the formative phase (Fig. 25.2). In addition to standard AP and optional axillary views, internal and external rotation views have also been recommended for identification and evaluation of deposits (4). Deposits become less dense and homogenous during the acute or resorptive phase and may become difficult to visualize with plain films. In addition, during the resorptive phase, a crescent of calcification may be seen in the overlying bursa due to rupture into the bursa. Serial radiographs are helpful in following the progression of the resolution of pathology (Fig. 25.3). Calcifications from arthropathies are associated with degenerative articular changes and are adjacent to the bony insertion and thus can be differentiated from calcific tendinitis.
Some associations between particular radiographic features and the phase of disease have been advanced. It has been suggested that during the formative or chronic phase, the deposit is homogenous, well defined, and dense (Fig. 25.2) and then becomes fluffy and heterogenous during the resorptive or acute phase (Fig. 25.3).
MRI and ultrasound have been used to visualize calcifications in those cases (10). MRI is rarely indicated, but when obtained, deposits appear as areas of decreased signal intensity on T1-weighted images, whereas on T2-weighted images they often show a perifocal band of increased signal intensity (Fig. 25.4). Ultrasound has been found to be more sensitive than plain radiographs by some authors, with 100% ultrasound compared with 90% radiographic identification of histologically proven calcific tendinitis. It should be noted, as with any ultrasound technique, that these results are very operator dependent.
Classification
Bosworth (4) divided deposits into three sizes, which he felt correlated with the increasing likelihood of clinical significance: small, measuring up to 0.5 mm; medium, measuring 0.5 to 1.5 mm; and large, measuring greater than 1.5 mm. Calcific tendinitis has also been described based on the duration of symptoms as acute (up to 2 weeks), subacute (3 to 8 weeks), and chronic (3 months or more) (5). The chronic presentations are likely to occur during the formative phase, whereas the acute phase occurs during resorption. Calcific tendinitis has also been described as localized or diffuse, with the diffuse form generally causing symptoms that are more severe and of longer duration. Rowe (6) described a chronic asymptomatic stage correlating with the gross finding of dry, powder-like calcific
deposits, a mild chronic pain correlating with the gross finding of soft toothpaste-like deposits, and a milky or creamy consistency in the extremely painful period. Harvie et al. (7) recommended classifying calcific tendinitis as idiopathic (type I) or secondary (type II) based on whether patients had an endocrine disorder. In their study, those with endocrine disorders (type II) had a higher rate of failure of conservative management leading to surgical intervention compared with those without an endocrine diagnosis (type I), with 47% surgical intervention required in type II versus 23% in type I patients.
deposits, a mild chronic pain correlating with the gross finding of soft toothpaste-like deposits, and a milky or creamy consistency in the extremely painful period. Harvie et al. (7) recommended classifying calcific tendinitis as idiopathic (type I) or secondary (type II) based on whether patients had an endocrine disorder. In their study, those with endocrine disorders (type II) had a higher rate of failure of conservative management leading to surgical intervention compared with those without an endocrine diagnosis (type I), with 47% surgical intervention required in type II versus 23% in type I patients.
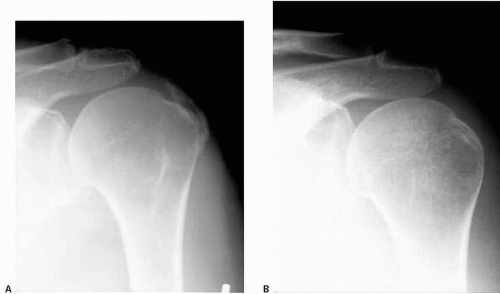 FIGURE 25.3. A, B: Serial AP radiographs taken 3 months apart demonstrating resorption of calcifications. A: the initial image. B: the same view taken 3 months later. |
Gartner and Heyer (1) created a radiographic classification, which is often referenced, with type I referring to a homogenous deposit on X-ray with a sharp outline, type II an inhomogenous structure with a sharp outline or a homogenous structure without a defined outline, and type III an inhomogenous structure without a defined outline. He reported that response to needling is related to the appearance of the deposit, with 85% of fluffy accumulations (type III) resorbed at 3 years versus only 33% of sharply defined calcifications (type I). Patte and Goutallier (8) also classified deposits radiographically.
A French classification system (9) that is commonly used is based on the radiographic appearance as well: type A is dense, rounded, and sharply delineated; type B is multilobular in appearance, radiodense, and with sharp outlines; type C is more radiolucent and heterogenous with irregular outlines; and type D has dystrophic calcific lesions at the tendon insertion, which many would say is separate from true calcific tendinitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree