Chapter 26 Bundle Branch Reentrant Ventricular Tachycardia
Pathophysiology
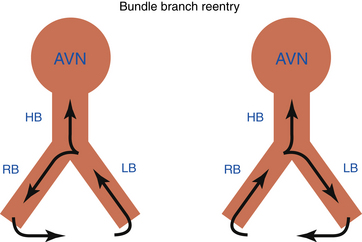
Bundle branch reentrant (BBR) ventricular tachycardia (VT) is a reentrant VT with a well-defined reentry circuit, incorporating the right bundle branch (RB) and left bundle branch (LB) as obligatory limbs of the circuit, connected proximally by the His bundle (HB) and distally by the ventricular septal myocardium (Fig. 26-1).
Single BBR beats can be induced in up to 50% of patients with normal intraventricular conduction undergoing electrophysiological (EP) study. The QRS during BBR can display either left bundle branch block (LBBB) or right bundle branch block (RBBB) when anterograde ventricular activation occurs over the RB or LB, respectively. The vast majority have LBBB configuration. BBR with an LBBB pattern can also occur occasionally during right ventricular (RV) pacing. This requires that the effective refractory period of the LB be longer than that of the RB, or that retrograde conduction over the RB be resumed after an initial bilateral block in the His-Purkinje system (HPS) (i.e., gap phenomenon). Left ventricular (LV) pacing does not seem to increase the yield of induction of BBR with RBBB morphology.1
In patients with normal intraventricular conduction, BBR is a self-limited phenomenon. The rapid conduction and long refractory period of the HPS prevent sustained BBR in normal hearts. Spontaneous termination of BBR most commonly occurs in the retrograde limb between the ventricular muscle and the HB.1 Sometimes, anterograde block can also occur, making refractoriness in the RB-Purkinje system the limiting factor. Continuation of BBR as a tachycardia is critically dependent on the interplay between conduction velocity and recovery of the tissue ahead of the reentrant wavefront. Two changes from normal physiology must occur for BBR to become sustained: (1) an anatomically longer reentrant pathway caused by a dilated heart, providing sufficiently longer conduction time around the HPS; and (2) slow conduction in the HPS caused by HPS disease.1 These two factors are responsible for sufficient prolongation of conduction time to permit expiration of the refractory period of the HPS ahead of the propagating reentrant wavefront.
Clinical Considerations
Epidemiology
Sustained BBR VT usually occurs in patients with structural heart disease, especially dilated cardiomyopathy. Idiopathic dilated cardiomyopathy is the anatomical substrate for BBR VT in 45% of cases, and BBR VT accounts for up to 41% of all inducible sustained VTs in this population. BBR VT can also be associated with cardiomyopathy secondary to valvular or ischemic heart disease, and has been reported with Ebstein anomaly, hypertrophic cardiomyopathy, and even in patients without structural heart disease other than intraventricular conduction abnormalities.2
In patients with spontaneous sustained monomorphic VT, the incidence of inducible BBR VT ranges from 4.5% to 6% in patients with ischemic heart disease to 16.7% to 41% in patients with nonischemic cardiomyopathy. BBR VT accounts for up to 6% of all forms of induced sustained monomorphic VT. Importantly, in patients with BBR VT, additional myocardial VTs occur in 25%.3 Of note, BBR VT is more frequently found in patients with VT clusters (up to 12.5%) than in patients with less frequent episodes of VT.4
Principles of Management
Associated myocardial VT occurs in approximately 25% of patients post ablation, and these patients continue to be at a high risk of sudden cardiac death. Therefore, implantable cardioverter-defibrillator (ICD) therapy is indicated for secondary prevention, and additional antiarrhythmic therapy is required for some patients. ICD implantation will also provide back-up pacing, which is frequently required post ablation secondary to the development of atrioventricular (AV) block or an excessively prolonged His bundle–ventricular (HV) interval. Implantation of a dual-chamber or biventricular ICD should be considered in these patients.
EP testing should be considered in patients with repetitive episodes of VT and dilated cardiomyopathy, history of cardiac valve repair or replacement, or QRS morphology during VT similar to sinus rhythm QRS. If sustained BBR VT is inducible during programmed stimulation, catheter ablation is recommended.4
Electrocardiographic Features
Baseline ECG
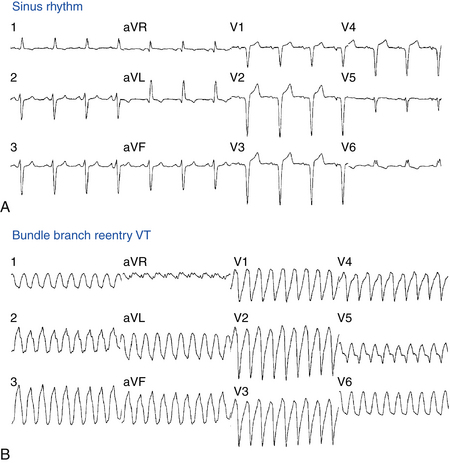
The baseline rhythm is usually NSR or atrial fibrillation (AF). Almost all patients with BBR VT demonstrate intraventricular conduction abnormalities. The most common ECG abnormality is nonspecific intraventricular conduction delay (IVCD) with an LBBB pattern and PR interval prolongation (Fig. 26-2). Complete RBBB is rare but does not preclude BBR as the mechanism of VT. Although total interruption of conduction in one of the bundle branches would theoretically prevent occurrence of BBR, an ECG pattern of complete BBB may not be an accurate marker of complete conduction block; a similar QRS configuration can be caused by conduction delay, rather than block, in the bundle branch. Occasionally, complete AV block may be observed.2
ECG during Ventricular Tachycardia
Twelve-lead ECG documentation of BBR VT is usually unavailable because the VT is rapid and hemodynamically unstable. The VT rate is usually 180 to 300 beats/min. QRS morphology during VT is a typical BBB pattern and can be identical to that in NSR. BBR VT with an LBBB pattern is the most common VT morphology, and it usually has normal or left axis deviation (see Fig. 26-2). In contrast to VT of myocardial origin, BBR with an LBBB pattern characteristically shows a rapid intrinsicoid deflection in the right precordial leads, suggesting that initial ventricular activation occurs through the HPS and not ventricular muscle. BBR VT with an RBBB pattern usually has a leftward axis, but it can have a normal or rightward axis, depending on which fascicle is used for anterograde propagation.1
Electrophysiological Testing
Baseline Observations during Normal Sinus Rhythm
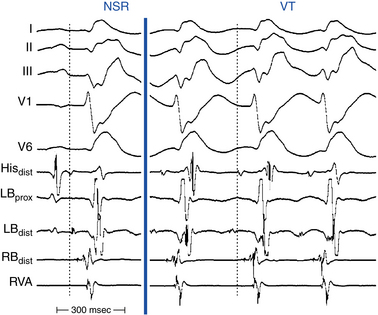
Conduction abnormalities in the HPS are almost invariably present and are a critical prerequisite for the development of sustained BBR, regardless of the underlying anatomical substrate (Fig. 26-3).5 The average HV interval is about 80 milliseconds (range, 60 to 110 milliseconds). Although some patients can have the HV interval in NSR within normal limits, functional HPS impairment in these patients manifests as HV interval prolongation or split HB potentials, commonly becoming evident during atrial programmed stimulation or burst pacing. Nonspecific IVCD with an LBBB pattern and PR interval prolongation are the most common abnormalities.2
Induction of Tachycardia
During RV pacing at a constant cycle length (CL) and during introduction of VES at relatively long coupling intervals, retrograde conduction to the HB occurs via the RB. At shorter VES coupling intervals, retrograde delay and block occur in the RB when its relative and effective refractory periods are encountered, respectively. When retrograde block occurs in the RB, the impulse propagates across the septum and retrogradely up the LB to the HB, producing a long V2-H2 interval. The LB would still be capable of retrograde conduction because of its shorter refractoriness and because of the delay associated with transseptal propagation. Further shortening of the coupling intervals is associated with increasing delay in LB conduction (i.e., increasing V2-H2 interval). Within a certain range of coupling intervals, increasing retrograde LB delay allows for the recovery of anterograde conduction via the RB, and another ventricular activation ensues, displaying a wide QRS with an LBBB pattern. This beat is called the “BBR beat” or “V3 phenomenon.”2
BBR is more likely to occur when the VES is delivered following pacing drives incorporating long to short CL changes as compared with constant CL drives, because of CL dependency of the HPS refractoriness. An abrupt change in CL (i.e., long to short) can result in a more distal site of retrograde block, and less concealment, along the myocardium-Purkinje-RB axis, which can allow sufficient recovery of excitability in the anterograde limb of the circuit (i.e., the RB-Purkinje-myocardium axis) for reentry to develop. In addition, earlier recovery of excitability along this axis, because of the more distal site of block and less concealment, is associated with a shorter H2-V3 interval in this reentrant beat.1
Procainamide, which increases conduction time within the HPS, especially in the diseased HPS, and, potentially, isoproterenol can facilitate induction of sustained BBR. In some patients, the arrhythmia can be inducible only with atrial pacing.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree