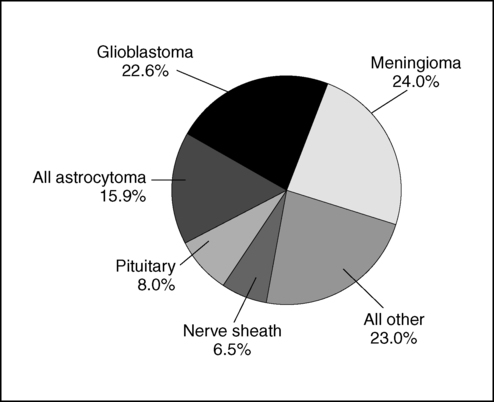
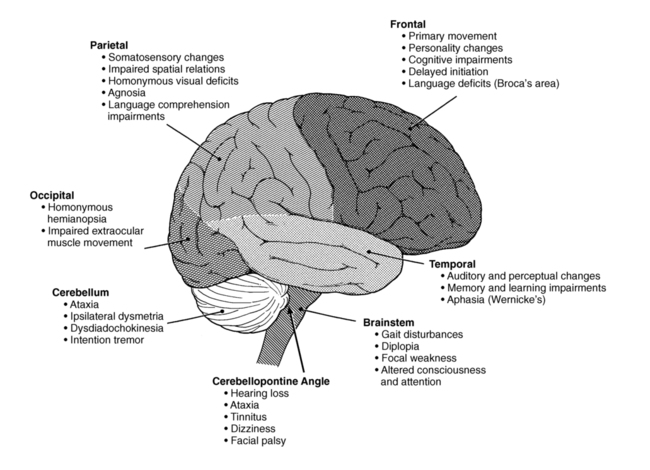
CORRIE J. STAYNER, PT, MS, RACHEL M. LOPEZ, PT, MPT, NCS and KARLA M. TUZZOLINO, PT, NCS After reading this chapter the student or therapist will be able to: 1. Identify the categories of primary brain tumors. 2. Recognize and interpret signs and symptoms of primary brain tumors specific to tumor location. 3. Recognize current diagnostic tests used to detect brain tumors. 4. Identity the types of medical and surgical management for brain tumors and how that management will affect functional movement. 5. Describe the side effects associated with the treatment of brain tumors and recognize their impact on therapeutic intervention. 6. Discuss the multiple considerations necessary to plan and execute an intervention program for the client with a brain tumor. 7. Recognize the emotional and psychosocial impact of the disease process on the client, the client’s support system, and the interdisciplinary team. The rehabilitation clinician serves many different populations, including clients with brain tumors. Despite the prognosis for limited survival associated with primary brain tumors, these individuals have shown progress in the rehabilitation setting similar to that noted in clients with diagnoses of stroke or traumatic brain injury.1–3 Advances in medical and surgical treatment for clients with cancer have resulted in improved survival rates and longer life expectancy. However, individuals are often faced with progressive impairments resulting from the disease process.2 These impairments may be physical or cognitive, or both, and require an interdisciplinary team approach to best facilitate the individual’s participation in a meaningful lifestyle. In addition, clinicians must recognize the psychological and emotional needs of the individual given this diagnosis and be sensitive and flexible in accommodating the patient’s feelings. Improved quality of life, especially the opportunity to return home, remains the ultimate goal of the rehabilitation process. The incidence of adult brain tumors is on the rise in the United States, with an estimated 62,930 new cases of primary benign or malignant brain and CNS tumors for 2010. The statistics for children include 4030 new cases for the same 12-month period, of which 2880 will occur in children younger than 15 years of age.4,5 The exact cause of the increase in incidence of brain tumors is not known. Studies suggest that the increase is the result of more tumors being diagnosed with improved tumor imaging, rather than an actual increase in the occurrence of malignant brain tumors.6,7 In the United States, brain tumors typically occur in two distinct categories of patients: (1) children aged 0 to 15 years and (2) adults in the fifth to seventh decades of life. In adults, white Americans have a higher incidence than black Americans, and in both pediatric and adult populations males are more frequently affected than females.8,9 In children, a primary brain tumor is now the most common cause of solid tumor cancer death in the 0- to 15-year-old age group and the second overall cancer after leukemia.5 The frequently occurring meningioma, typically benign, accounts for 33.8% of all primary brain tumors. Glioblastoma multiforme, a malignant tumor, accounts for 17.1% of adult primary tumors (Figure 25-1).5 The largest percentage of childhood tumors (17%) are located in the frontal, temporal, parietal, and occipital lobes of the brain, followed by 16% in the cerebellum and 11% in the brain stem.5 The etiology of brain tumors remains unclear. Theories suggest that heredity is a contributing factor, but studies show familial incidence can be explained by a common toxic or infectious exposure.10,11 Research indicates an association, but not a causal relationship, linking brain tumors to certain chemicals and materials (petrochemicals, organic solvents, rubber). These materials are frequently found in specific occupations, such as farming and manufacturing. Electromagnetic field exposure is associated with an increased incidence of brain tumor.12 Ionizing radiation, used therapeutically in high doses to treat tumors, was found to have a causal relationship to the development of a second brain tumor.13 The World Health Organization (WHO) first published a universal classification system for CNS tumors in 1979. This system classifies tumors according to their microscopic characteristics and has been accepted as the universal method for the classification of brain tumors.14,15 Gliomas are primary tumors that arise from supportive tissues of the brain and are frequently located in the cerebral hemispheres. These tumors may also occur in the brain stem, optic nerve, and spinal cord. In children, the cerebellum is a primary location for gliomas.13,16 Gliomas have four primary categories and are classified by their predominant cellular components: astrocytomas and oligodendrogliomas originate from glial cells, ependymomas from ependymal cells, and medulloblastomas from primitive cells.17 Astrocytomas are derived from astrocytes, which are star-shaped glial cells, and are the most common primary brain tumor in adults and children.18 Astrocytomas vary in morphology and biological behavior, from those that are diffuse and infiltrate surrounding brain structures, to those that are circumscribed with a decreased likelihood of progression. Astrocytomas are typically found in the cerebrum, originating in the frontal lobe in adults, and in the cerebellum in children. In adults the primary age at onset is typically in the third to fifth decades of life.7,14 Astrocytomas are further classified into four grades: pilocytic, well-differentiated, relatively benign low-grade tumors most common in childhood and young adults (grade I); diffuse, well differentiated, low-grade tumors (grade II); anaplastic, high-grade tumors (grade III); and glioblastoma multiforme, high-grade (grade IV). The higher the grade, the poorer the prognosis.7 Low-grade tumors (grade II) grow slowly and are typically subtotally resected through surgery when accessible, whereas grade I tumors occur primarily in children and are typically cured with complete surgical resection. As a result of incomplete resections, recurrence is common.7 As these tumors recur, their form and structure often change to that of an anaplastic astrocytoma or glioblastoma.17 Anaplastic, midgrade (grade III) tumors grow rapidly, typically carry malignant cell traits, and routinely progress toward glioblastoma multiforme tumors.14 Astrocytomas are typically treated with surgery, radiation therapy, and chemotherapy, depending on the grade, location of the tumor, age of the patient, and Karnofsky performance scale score (Table 25-1).7,16,19 Pilocytic astrocytomas carry a 5-year survival rate of 94%; however, patients with grade III astrocytomas have a 5-year survival rate of only 27%.5 TABLE 25-1 KARNOFSKY PERFORMANCE STATUS SCALE Adapted from Karnofsky DA, Burchenal JH: The clinical evaluation of chemotherapeutic agents in cancer. In Macleod C, editor: Evaluation of chemotherapeutic agents, New York, 1949, Columbia University Press. Glioblastoma multiforme is the distinct name given to the highly malignant grade IV astrocytoma. These tumors grow rapidly, invade nearby tissue, and contain highly malignant cells. Glioblastomas are predominantly located in the deep white matter of the cerebral hemispheres but may be found in the brain stem, cerebellum, or spinal cord. Fifty percent of these tumors are bilateral or occupy more than one lobe of a hemisphere.7,14,16 Glioblastomas account for 17% of all primary brain tumors. They are most common in older adults and uncommon in children, with males having a 1.6:1 incidence rate over females.5 The medical prognosis is poor for persons with glioblastoma: less than 33% survive more than 1 year and less than 5% survive 5 years.5 The most important prognostic variables are age, tumor histology, and postoperative score on the Karnofsky performance status scale. These tumors are treated by surgical resection, radiation therapy, stereotactic radiosurgery, and chemotherapy.7 Oligodendrogliomas are slow-growing but progressive tumors that typically develop over a period of several years, with 50% involving multiple lobes. Fifty percent of these tumors occur in the frontal lobe, 42% in the temporal lobe, and 32% in the parietal lobe. Many clients have seizures as the only clinical manifestation of the tumor.14,20,21 Oligodendrogliomas typically appear in the fourth to sixth decades of life, and the ratio of affected males to females is 2:1.22 The prognosis with oligodendrogliomas varies considerably and is dependent on age at diagnosis and tumor grade. Positive prognostic indicators have been age at onset of less than 40 years and a tumor grade of I or II. These patients have a 5-year survival rate of 79% and 10-year survival rate of 64%.5 The 5-year survival rate decreases to 47% with anaplastic oligodendroglioma.5 Treatment is dependent on symptoms and ranges from observation and seizure control with anticonvulsant drugs to surgical resection followed by radiation and chemotherapy.7,17,20 Negative prognostic indicators include age at onset over 40 years, hemiparesis, and cognitive changes.23 Ependymomas and ependymoblastomas are tumors arising from ependymal cells, cells that line the ventricles of the brain and central canal of the spinal cord.16,17 These cells have glial and epithelial characteristics. The tumors grow into the ventricle or adjacent brain tissue. The most common site is the fourth ventricle (70% originate here); they occur less frequently in lateral and third ventricles.22 For supratentorial tumors, the age at onset is evenly distributed across the life span, whereas tumors originating in the fourth ventricle more frequently occur in childhood.22 Ependymomas are primarily treated with surgical resection followed by radiation therapy, but chemotherapy is also used.7,16,17,22 These tumors frequently recur, and prognosis is dependent on the success of resection, with a 5-year survival rate approaching 82%.5 Medulloblastomas are malignant embryonal tumors thought to arise from primitive neuroectodermal cells, specifically pluripotential stem cells that have been prevented from maturing to their normal growth-arrested state. The exact cell of origin, however, is still unknown.22 These tumors are typically located in the posterior fossa, originating laterally in the cerebellar hemispheres in young adults and in the vermis in children.14 Medulloblastomas typically grow into the fourth ventricle, blocking cerebrospinal fluid (CSF) flow, causing hydrocephalus and increased intracranial pressure (ICP).7 These tumors primarily occur in children, accounting for 20% of childhood (0 to 19 years) brain tumors, with the most common age of onset being 4 to 8 years old; they are more prevalent in males than females.22 An overall 5-year survival rate of 61% has been noted among adults and children; the most common treatment is surgery followed by radiation and chemotherapy.5,14,22 Meningiomas are slow-growing tumors that primarily originate from cells located in the dura mater or arachnoid membrane and account for 33% of reported brain tumors.5,18,22 Frequently these tumors are found incidentally during imaging studies or at autopsy.24 Approximately 25% of patients are symptomatic when diagnosed.18,24,25 Meningiomas are classified by their cytoarchitecture and genetic origin into four categories: (1) meningothelial or syncytial, (2) fibroblastic, (3) angioblastic variants, and (4) malignant.22 The incidence increases with age, and they occur in females at a 2:1 ratio over males.3,5,26,27 Resectable tumors are primarily treated by surgery, and recurring tumors are treated with surgery, radiation therapy, or stereotactic radiosurgery.17 Patients with nonmalignant meningiomas have a 5-year survival rate of 70% versus a 5-year survival rate of 55% with malignant meningiomas.5,28 Pituitary adenomas are benign epithelial tumors originating from the adenohypophysis of the pituitary gland and frequently encroach on the optic chiasm.14,17,22 These tumors are characterized by hypersecretion or hyposecretion of hormones.7,16 Age at onset spans all ages, but pituitary adenomas are rare before puberty.7 The female-to-male ratio of incidence is 3:1.5 These tumors are primarily treated by surgical resection and drug therapy.7,16,17 Prognosis is related to size and tumor cell type, with a 5-year survival rate of 70%.5 Schwannomas are encapsulated tumors composed of neoplastic Schwann cells that can arise on any cranial or spinal nerve.16,17 The eighth cranial nerve is the cranial nerve usually involved, and a schwannoma here is called an acoustic neuroma.14 Acoustic neuromas produce otological, focal or generalized neurological impairments, depending on the location of the tumor. These tumors are typically located in the internal auditory canal but may extend into the cerebellopontine angle.7,22 These tumors are frequently treated by surgical resection, but stereotactic radiosurgery is increasing in popularity as an alternative method of treatment.16,29,30 The prognosis for patients with these tumors is good, yet complications can result from treatment, including facial paralysis, deafness, and equilibrium impairments. Resulting activity limitations after surgery vary depending on the size and location of the tumor. Currently these tumors rarely result in death, and with the increasing use of noninvasive procedures the eighth cranial nerve is more frequently being preserved.30 Primary CNS lymphoma represents only 1% of intracranial tumors, although the incidence has significantly increased in the last two decades. This increase may be a result of its frequency in individuals with acquired immunodeficiency syndrome (AIDS) and other immunosuppressed states. Surprisingly, there is an increased frequency in immunocompetent persons; however, no evidence-based explanation has been found. These lymphomas have a slightly higher incidence in men and peak in the fifth through seventh decades of life, or in the third and fourth decades in individuals with AIDS. The tumor cells are similar in histology to systemic non-Hodgkin lymphoma cells, but it is uncertain how this tumor arises, as the CNS lacks lymphatic tissue.22,31 The tumor may be solitary or multifocal, forming a poorly defined mass that may be difficult to distinguish from an astrocytoma.22 Although CSF cytology may be diagnostic, stereotactic brain biopsy is often needed for definitive diagnosis.31 Primary brain lymphomas may arise in the cerebrum, cerebellum, or brain stem; however, 60% occur in the cerebral hemisphere. More frequently, presenting symptoms are behavioral and personality changes, confusion, dizziness, and focal cerebral signs rather than headache and other signs of increased ICP.22 Surgical resection is typically ineffective because of the deep location of these tumors. Cranial irradiation and corticosteroids frequently yield a partial or complete response; however, the tumor recurs in 90% of these individuals.22 A more recent favorable treatment includes the administration of intravenous methotrexate and leucovorin over 2- to 3-week intervals with corticosteroids to control neurologic symptoms.22 CNS lymphoma carries a poor prognosis, with only 27% of patients surviving longer than 5 years.5 Metastatic brain tumors originate from malignancies outside of the CNS and spread to the brain, typically through the arterial circulation.7,22 Approximately 25% of individuals with systemic cancer develop metastatic brain tumors, approximately 80% in cerebral hemispheres and 20% in the posterior fossa.22,31,32 One third of brain metastases originate in the lung, followed by the breast, skin, gastrointestinal tract, and kidneys in order of frequency. The frontal lobe is the most common site for metastatic disease from these systemic sources. Common clinical manifestations of metastatic brain tumors are similar to those of gliomas, including seizures, headache, focal weakness, mental and behavioral limitations, ataxia, aphasia, and signs of increased ICP.22 Treatment for these tumors is tailored to the individual and dependent on the management of the systemic disease, the accessibility of the lesion, and the number of lesions.32,33 Current treatment regimens use combinations of corticosteroids, brain irradiation, surgical intervention, and chemotherapy.22 The prognosis varies, with positive prognostic indicators including the Karnofsky performance scale score of 70 or greater, age 60 years or younger, remission or resolution of the primary cancer, and metastases located in the brain only.32,33 The average survival with treatment is approximately 6 months but varies widely and is affected by the extent of other systemic metastases. With some radiosensitive tumors, survival increases to 15% to 30% for 1 year and 5% to 10% for 2 years.22 The clinical manifestation of a brain tumor can range from a decreased speed in comprehension or a minor personality change to progressive hemiparesis or seizure, depending on the type and site of the tumor. Patients with brain tumors typically have headaches, seizures, nonspecific cognitive or personality changes, or focal neurological signs.22,34 The presenting sign in some may be a general sign, a specific neurological symptom, or a combination of both. 1. The headache that interrupts sleep or is worse on waking and improves throughout the day 2. The headache that is elicited by postural changes, coughing, or exercise 3. The headache of recent onset that is more severe or of a different type than usual 4. The new onset of headache in a previously asymptomatic person 5. The headache associated with nausea and vomiting, papilledema, or focal neurological signs1,7 The mechanism of the headache is not clearly understood but may be related to local swelling, distortion of blood vessels, direct invasion of the meninges, and increased ICP. When the tumor has grown to a volume large enough to cause compression and displacement of the brain, the onset and severity of the headache seem to correlate with changes in ICP.35 With increased ICP, a bifrontal or bioccipital headache is present regardless of the tumor location.22,36 Seizure activity is the presenting symptom in one third of cases and is present in 50% to 70% of cases at some stage of the disease.13,34 Approximately 10% to 20% of adults with new-onset seizure activity have brain tumors. Seizures are usually focal but may become generalized and cause loss of consciousness.37 Frontal lobe gliomas produce seizures in 59% of all cases. The percentages of patients exhibiting seizures from gliomas in other lobes are as follows: parietal, 42%; temporal, 35%; and occipital, 33%.34 Altered mental status is the initial symptom in 15% to 20% of individuals with brain tumors and is frequently present at the time of diagnosis. Mental status changes can range from subtle changes in concentration, memory, affect, personality, initiative, and abstract reasoning to severe cognitive problems and confusion.34 Subtle changes may be incorrectly attributed to worry, anxiety, or depression.22 Changes in mentation are common with frontal lobe tumors and in the presence of elevated ICP. Increased ICP causes drowsiness and decreased level of consciousness, which can progress to stupor or coma if treatment is not initiated.34 The incidence of papilledema, swelling of the optic nerve, is less frequent today because brain tumors are being diagnosed earlier with the use of sensitive imaging techniques. Papilledema is associated with symptoms of transient visual loss, especially with positional changes, and reflects evidence of increased intracranial hemorrhage transmitted through the optic nerve sheath. It is more common in children and with slow-growing tumors and posterior fossa tumors.34 Other, less common symptoms are vomiting and frank positional vertigo, usually accompanying tumors found in the posterior fossa.22 Certain clinical features are related to functional areas of the brain and thus have a specific localizing value in medically diagnosing a brain tumor.34 Therefore it is essential that clinicians be familiar with the lobes of the brain and their distinct functions to effectively manage the impairments resulting from the tumor (Figure 25-2). These symptoms may vary among individuals and result in activity limitations that range from mild to severe.35 The frontal lobe is responsible for motor functioning, initiation of action, and interpretation of emotion, including motor speech, motor praxis, attention, cognition, emotions, intelligence, judgment, motivation, and memory.38,39 Therefore frontal lobe tumors may result in movement disorders such as hemiparesis, seizures, aphasia, and gait difficulties. Initially the tumor may be clinically silent. As the tumor grows, however, there may be personality changes, including disinhibition, irritability, impaired judgment, and lack of initiation.34 Bifrontal disease usually associated with infiltrative gliomas and primary CNS lymphomas may cause bilateral hemiplegia; spastic bulbar palsy; severe cognitive impairment; emotional lability; dementia; and prominent primitive grasp, suck, and snout reflexes.35 The parietal lobe processes complex sensory and perceptual information related to somesthetic sensation, spatial relations, body schema, and praxis. General symptoms of a parietal lobe tumor include contralateral sensory loss and hemiparesis, homonymous visual deficits or neglect, agnosias, apraxias, and visual-spatial disorders. If the dominant parietal lobe is involved, aphasia and seizures may be present. With nondominant parietal lobe involvement, contralateral neglect and decreased awareness of impairments can commonly be found.26,34,36 The occipital lobe is the primary processing area of visual information. Therefore lesions of the occipital lobe often result in dysfunction of eye movement and homonymous hemianopsia. If the parieto-occipital junction is involved, visual agnosia and agraphia are often present. Although less common, visual seizures may be present, characterized by lights, colors, and formed geometric patterns.33,34,36 Bilateral occipital tumors may cause cortical blindness.35 The temporal lobe is responsible for auditory and limbic processing. Anterior temporal lobe lesions may be clinically silent until they have become quite large, resulting in seizures. If the lateral hemispheres are involved, auditory and perceptual changes may occur. When the medial aspects of the lobe are involved, changes in cognitive integration, long-term memory, learning, and emotions may be seen. When the dominant temporal lobe is involved, aphasia may be present. Anomia, agraphia, acalculia, and Wernicke aphasia, characterized by fluent, nonsensical speech, are specific to left temporal lobe lesions.33,34,36 In comparison with bifrontal tumors, bitemporal tumor involvement is rare and causes memory deficits and possible dementia.35 The cerebellum is responsible for coordination and equilibrium.26(Refer to Chapters 21 and 22A.) The most common symptoms of cerebellar tumors in adults include headache, nausea and vomiting in 40% of cases, and ataxia in 25% of cases. Lesions of the midline cause truncal and gait ataxia, and lesions of the hemispheres cause unilateral appendicular ataxia, most commonly seen in the upper extremities. Lesions of either hemisphere may cause ipsilateral dysmetria, dysdiadochokinesia, and intention tremor. If the tumor involves the cerebellopontine angle, hearing loss, headache, ataxia, dizziness, tinnitus, and facial palsy may occur. If the tumor invades the meninges at the foramen magnum or increased ICP causes cerebellar tonsil herniation, nuchal rigidity and head tilt away from the lesion may be seen. Abnormal posturing of the head is observed in children but not adults.35 Because the cerebellum is located in an extremely confined space, even minimal increases in pressure can cause death from cerebellar tonsil herniation.7,34,35,40 The brain stem, which communicates information to and from the cerebral cortex via fiber tracts, controls basic life functions. The reticular formation specifically controls consciousness and attention. Even small changes in tumors invading or compressing the brain stem can lead to death or devastating signs and symptoms. Symptoms of a brain stem tumor have an insidious onset and may include gait disturbances, diplopia, focal weakness, headache, vomiting, facial numbness and weakness, and personality changes.7 If the dorsal midbrain is involved, Parinaud syndrome, characterized by loss of upward gaze, pupillary areflexia to light, and loss of convergence, may be seen. If the reticular system of the pons and medulla is involved, symptoms of apnea, hypoventilation or hyperventilation, orthostatic hypotension, or syncope may occur.7,40 The pituitary gland is an endocrine gland that secretes hormones that regulate many bodily processes. Pituitary tumors are typically large and affect pituitary function by compressing its structure or hypersecreting hormones. An enlarging tumor causes a loss of pituitary function and decreases hormone secretion, resulting in pituitary disorders specific to the type of hormone involved (e.g., Cushing disease, hypothyroidism, Addison disease, diabetes). As the tumor enlarges it may invade or compress nearby structures. Lateral extension involving the third and fourth cranial nerves causes diplopia; fifth cranial nerve involvement causes ipsilateral facial numbness; and internal carotid artery occlusion causes cerebral infarction. Upward extension is more common and may compress the optic chiasm, hypothalamus, or third ventricle. Downward extension may compress the sphenoid sinus, typically without clinical signs.7 Advances in research and imaging technology have greatly improved brain tumor medical diagnosis. When a physician suspects a brain tumor, many specialized tests may be used to gather clinical, radiological, pathological, and laboratory information to confirm the diagnosis.31,34 A clinical diagnosis consists of information the physician gathers during a comprehensive evaluation. First, a thorough medical history, including the specific nature of signs and symptoms, must be obtained. A neurological examination is then performed to test reflexes and assess visual, cognitive, sensory, and motor function.40 If the presence of a brain tumor is suspected after the neurological examination, the next diagnostic step, tumor imaging, is warranted.22 The modern era of CNS imaging began with the introduction of computed tomography (CT) in 1973 and with magnetic resonance imaging (MRI) in 1979.7 The availability of sensitive imaging allows for earlier tumor detection and has revolutionized the diagnosis and management of brain tumors.34,41 Tumor imaging has continued to develop and can be classified into three categories: static, dynamic, and computer integration imaging. Each type of scan shows different features and function of the brain; therefore several scans may be needed for an accurate diagnosis.16 Static neurological imaging includes CT and MRI, which are noninvasive techniques that provide accurate anatomical and functional analysis of intracranial structures.34 CT uses ionizing radiation, thin bands of x-rays, to produce images of slices of brain tissue.31 It was the first brain imaging technique to allow determination of tumor size. Contrast enhancement helps to identify isodense tumor from surrounding parenchyma, hypodense lesions in edematous areas, and optimal sites for tumor biopsy.7,34 After surgical intervention, CT can be used to confirm the proper tissue biopsy site and determine the success of tumor resection. Although MRI has become the preferred method, CT scanning offers lower cost, a shorter scanning time, and a more sensitive method to detect calcification and bony involvement.22 MRI is the initial diagnostic imaging procedure of choice. MRI uses magnetic fields rather than ionizing radiation and is superior to CT scanning in detecting and localizing brain tumors, as well as evaluating edema, hydrocephalus, or hemorrhage.22,34,35 CT scans can miss structural lesions, especially posterior fossa tumors and low-grade gliomas.37 MRI is a more sensitive imaging modality than CT for identifying lesions and margin abnormalities by providing greater anatomical detail with thin slices and multiplanar images. With MRI, different signal intensities differentiate between normal brain and tumor. Contrast enhancement with gadolinium sharpens the definition of a lesion.7,22,36 Under certain conditions, MRI enhanced with gadolinium can distinguish between tumor and edema. However, not all high-grade astrocytomas enhance with gadolinium, and MRI signals may imitate imaging abnormalities seen in low-grade astrocytomas or nonmalignant conditions. MRI also cannot accurately predict tumor type or grade of malignancy, for which surgical biopsy is necessary.7,34 Dynamic functional imaging includes positron emission tomography (PET), single-photon emission CT (SPECT), magnetic resonance spectroscopy (MRS), and functional MRI. PET is a noninvasive technique using a cyclotron and specific isotopes to obtain dynamic information about the metabolism and physiology of the brain tumor and the surrounding brain tissue. PET scans using radioactive markers to measure glucose metabolism can be useful in determining the grade of primary brain tumors and in differentiating tumor regrowth from radiation necrosis.9,36,42 PET can also be helpful in studying the metabolic effects of chemotherapy, radiation therapy, and steroids on the tumor.34 However, PET is expensive and less reliable in patients treated heavily with chemotherapy and radiation therapy.7,22 SPECT is a functional imaging technique evolved from PET and uses isotopes without cyclotron to assess cerebral blood flow and determine tumor location.7,22,34 SPECT is used to identify high- and low-grade tumors and to differentiate between tumor recurrence and radiation necrosis.7,34 SPECT is used preoperatively with static imaging to localize the highest metabolic area within tumor for biopsy. Although SPECT is a less sensitive method of obtaining physiological information on brain tumors, it is more readily available and less expensive.7
Brain tumors
An overview of brain tumors
Incidence and etiology
Classification of tumors
Primary brain tumors

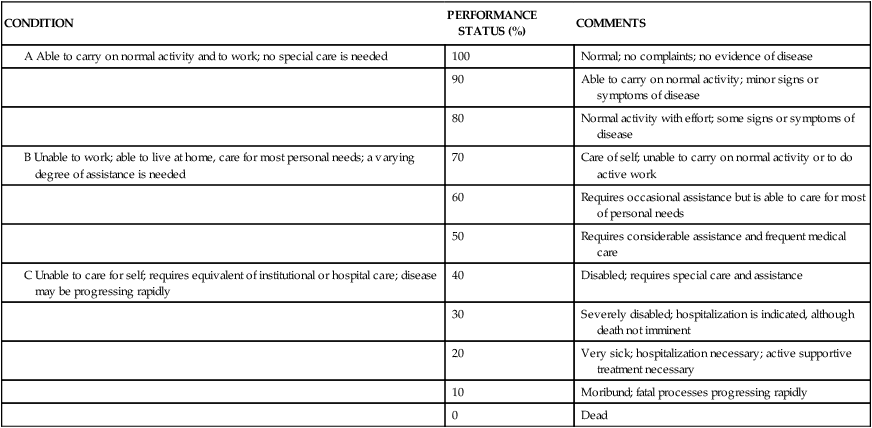
CONDITION
PERFORMANCE STATUS (%)
COMMENTS
100
Normal; no complaints; no evidence of disease
90
Able to carry on normal activity; minor signs or symptoms of disease
80
Normal activity with effort; some signs or symptoms of disease
70
Care of self; unable to carry on normal activity or to do active work
60
Requires occasional assistance but is able to care for most of personal needs
50
Requires considerable assistance and frequent medical care
40
Disabled; requires special care and assistance
30
Severely disabled; hospitalization is indicated, although death not imminent
20
Very sick; hospitalization necessary; active supportive treatment necessary
10
Moribund; fatal processes progressing rapidly
0
Dead
Secondary brain tumors: metastatic brain tumors
Signs and symptoms
General signs and symptoms
Specific signs and symptoms
Medical diagnosis of disease or pathology
Clinical diagnosis
Radiological diagnosis
Static imaging.
Magnetic resonance imaging.
Dynamic imaging.
Single-photon emission computed tomography.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Brain tumors
Only gold members can continue reading. Log In or Register to continue