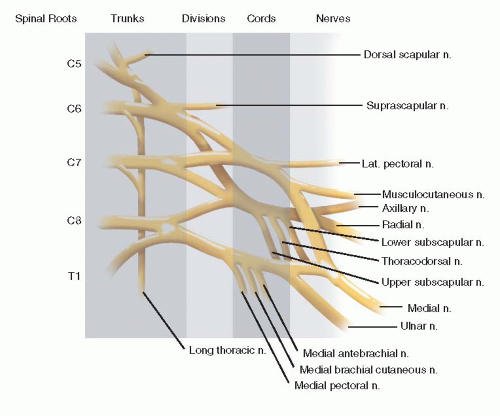
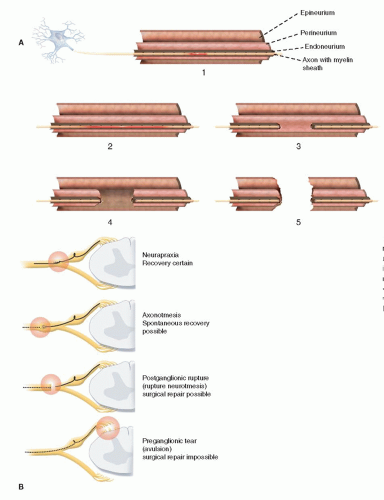
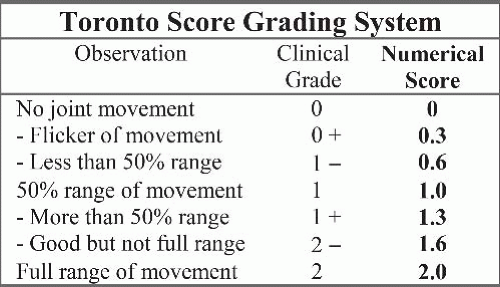
How common is brachial plexus birth palsy (BPBP)?
Can we define the cause?
How do you distinguish an avulsion injury from an extraforaminal rupture?
What is the expected outcome by natural history?
What are the indications for nerve surgery?
What techniques are most commonly used for nerve reconstruction?
What are the expected outcomes and differences from both avulsion and extraforaminal reconstructions?
foramina will be empty. Distal to the neuroma, depending on the size of the neuroma and extent of injury, more normal individual divisions, cords, and/or nerves are isolated. Each situation is different and, accordingly, recognized, dissected, and recorded. The simple goal is to provide healthy neural input to affected muscles. The method by which this is achieved varies from case to case.
include congenital differences, central nervous system or spinal cord pathology, and infection.
the brain-spinal cord axis and the peripheral nerve muscle axis at each avulsion level.
 FIGURE 20-3 Horner syndrome with ptosis, miosis, enophthalmos, and anhidrosis. There is a high correlation between an avulsion injury and the presence of a Horner syndrome. |
 FIGURE 20-4 Typical posture of elbow extension (absent biceps), wrist flexion (absent wrist extensors), and digital flexion (absent finger extension). |
long-term economic cost and outcome. We still do not have the definitive answers that can be applied to any given case. This ambiguity and lack of consensus complicates life for parents trying to decide what is best for their child’s future. The trends, however, are clear, and prospective study results are coming.
Table 20.1 Treatment recommendations in obstetric palsy | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Also, in infants there is a reasonable chance of some recovery of hand function. However, even the most experienced surgeons are challenged when there is a mismatch between what is needed and what is available for reconstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree