Bone tumors are those conditions of the skeletal system that are neoplastic or could be mistaken for a neoplastic condition on the basis of radiographic or pathologic evidence (
1). In the mid-1920s, Codman published the first articles of the registry of bone sarcoma. These articles described radiographic characteristics associated with specific bone tumors. Many of the early principles, such as evaluation of the bone-tumor interface and the presence of triangular periosteal reactions (Codman triangle), have stood the test of time and are still relied on to assess these osseous lesions (
2,
3). With the routine use of ancillary imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) in the evaluation of musculoskeletal lesions, in many instances the approach to bone tumors and tumor-like lesions will entail use of advanced imaging studies. Among their advantages, both of these modalities offer sectional imaging and have improved contrast resolution versus plain-film imaging. Nevertheless, CT, MR, radionuclide, positron emission tomography (
18FDG-PET), and ultrasonic scanning are indeed “ancillary” considerations. That is to say, for a suspected primary bone tumor, the plain-film radiograph is still the screening and/or first imaging modality of choice.
Clearly, CT and MRI are now routinely ordered by practitioners, many times intuitively, although sometimes as a “reflex.” However, for grossly benign-appearing lesions with plain-film studies, CT or MRI may not be necessary unless surgical intervention is contemplated and/or greater anatomic detail is required (
4). With respect to this latter point, CT imaging does indeed enjoy some “natural” indications when it comes to pedal imaging. Lesions located in bones with complex anatomic shapes and overlappings (e.g., the tarsal bones) typically require cross-sectional imaging to fully clarify their osseous extent and soft tissue involvement. In addition, many pedal lesions are statistically of smaller size than their counterparts in the extremity long bones. As a logical extension of plain-film radiographic imaging, CT scanning is generally superior in demonstrating intracortical lesions, subtle cortical disruptions and/or erosive changes, the extent of internal lysis (especially in tarsal bones), and the presence of intralesional calcification(s). Moreover, CT scanning is no longer limited in its ability to provide high-quality sagittal plane reconstructions of the foot. Newer developments such as multichannel, multidetector, and spiral/helical scanning technology now allow for clear reformatted sagittal plane pedal images as well as volumetric data acquisition, which can facilitate three-dimensional reconstruction.
Unlike CT scanning, MR imaging is not based upon differential x-ray beam attenuation within matter and provides cross-sectional imaging, but with markedly superior contrast resolution. This is especially useful in determining the
extent of bone tumors and the degree of soft tissue involvement. This information, in turn, is often vital for overall tumor staging (e.g., Enneking system, covered elsewhere in this text), which ultimately helps guide appropriate therapy and surgical management. MRI is, indeed, the modality most typically used for staging lesions of the extremities (
4,
5,
6 and
7).
As noted before, CT or MRI may not be necessary for benign-appearing lesions unless surgery is anticipated or greater anatomic detail is required (
4). Conversely, when routine radiographic findings are indeterminate or the lesion has a more aggressive appearance and/or is considered potentially malignant, ancillary imaging studies are typically required. Although radionuclide imaging is a consideration in this scenario, because of MRI’s improved anatomic detail and sensitivity, it is now generally preferred over radionuclide studies. Most notably, MRI is sensitive to the early hematogenous dissemination of a tumor to bone marrow before reactions in adjacent bone are detectable on
99mTc bone scans. In other words, MRI’s sensitivity to subtle changes in marrow exceeds bone scanning in the detection of osseous metastases.
Although there is significant lack of meta-analyses in the current literature with respect to indications for adjunctive imaging for suspected bone tumors, there is some direction available. According to the most recent (2008) American College of Radiology Appropriateness Criteria, which is dedicated to providing evidence-based guidelines to practitioners, in the scenario of a suspected primary bone tumor, MRI is most highly ranked (i.e., most appropriate or indicated) when (a) radiographic findings are suspicious for malignant characteristics or (b) when persistent symptoms exist with negative radiographs. Conversely, both MRI and CT score low as appropriate studies when radiographic features are definitely benign. In addition, CT scanning is preferred over MRI for imaging of suspected osteoid osteoma (
4).
To be sure, there are instances where MR imaging can lead the practitioner to the most probable diagnosis (e.g., lipoma). Unfortunately, however, most bone (and soft tissue) tumors present with nonspecific MR features, thereby limiting its usefulness as a stand-alone study. However, when combined with plain-film radiographic imaging, its overall diagnostic accuracy can significantly improve. As an example, Mahnken et al (
8) found that conventional radiography and MRI each demonstrated a sensitivity of 76.4% and 77.8% with a specificity of 55.0% and 66.7%, respectively. With their combined use, the sensitivity and specificity increased to 82.6% and 70%, respectively.
In light of the foregoing discussion, it should be apparent that, despite an array of impressive technologic imaging options, one must keep a proper perspective regarding their roles. In the scenario of a suspected bone tumor, the initial evaluation of the plain-film radiograph most often provides the information critical to correct interpretation and therefore integral to the process of formulating a working diagnosis and treatment plan. The practitioner is challenged to evaluate and/or classify (a) the pattern of osseous lysis, (b) contour and shape, (c) likely epicenter/location, (d) periosteal reaction, and (e) presence or absence of tumor matrix. These findings,
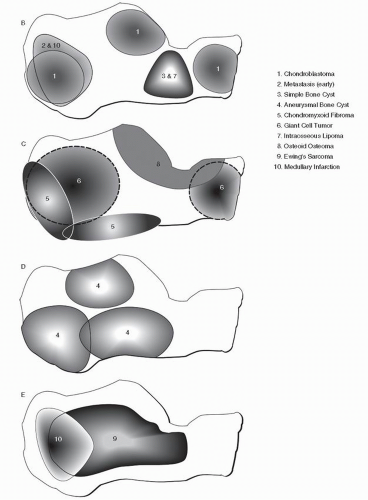
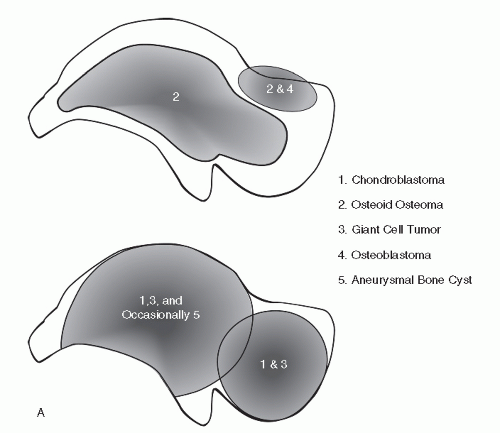
when weighed against pertinent clinical/epidemiologic data and behavioral trends, can afford the practitioner the ability to indeed arrive at a most likely diagnosis. However, there is nothing easy about this, and without a radiologic foundation rooted in the basics of delineating benign from malignant osseous lesions, all will be for naught. If there are some pragmatic aids or “revelations” in the plain-film radiographic interpretation of the foot and ankle bone tumors, they most likely lie with the development of preferential location maps of specific bone tumors for several major pedal bones, specifically the talus and calcaneus (
Fig. 93.1) (
9,
10). This data can then be weighed along with demographics and specific radiographic features to help arrive at a most probable diagnosis. Identifying trends for bone tumors of the phalanges, metatarsals, and midfoot bones has proven to be problematic. With respect to the phalanges and metatarsals, the early appearance of the lesion is often not encountered and the epicenter is uncertain. With respect to the midfoot bones, the exceedingly rare occurrence of virtually all bone tumors in the lesser tarsal bones currently does not facilitate reliable mapping of these bones.
APPROACH
Although primary bone tumors of the foot and ankle are rare, the possibility of neoplasm establishes a need for a systematic approach to radiographic evaluation of osseous lesions. In a patient with a neoplastic process, the clinical examination (history and physical) is often nonspecific. Thus, the accuracy and completeness of the initial radiographic evaluation are important steps in the overall management of this patient. Precise identification of a bone tumor is often an unrealistic goal. More to the point, one is challenged to formulate an appropriate differential diagnostic list, from which bone tumors may be ranked most likely to least probable based upon considerations presented in the remainder of this section. Perhaps most importantly, estimation of malignant potential can be extrapolated from the same analysis. For those lesions with predominantly benign-appearing features, serial radiographs at regular intervals should be employed to monitor any changes and are generally not necessary after 2 years without progression or clinical change. Any suspicion of malignancy mandates further workup.
The literature abounds with articles, chapters, and texts written on assessing bone tumors (
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40 and
41). The intent of this chapter is to provide the reader with a disciplined approach to a possible bone tumor of the foot and ankle. The first part of this chapter focuses on evaluation of specific radiographic parameters such as periosteal reactions and matrix patterns. This is followed by descriptions of specific bone tumors. An understanding of fundamental radiographic principles coupled with basic
knowledge of specific tumors will increase the accuracy of the initial differential diagnosis.
A classification system may be helpful to avoid overlooking any possible diagnosis. The following classification system, modified from Moser and Madewell (
25), divides all bone tumors into six categories (
Table 93.1). Each category represents the benign and malignant varieties of a specific tissue type. Discussion of every neoplastic variant that affects the foot and ankle would be cumbersome; this text is confined to entities that occur in the foot and ankle on a more frequent basis.
PRIMARY VERSUS SECONDARY NEOPLASIA
Metastatic osseous lesions occur more frequently than primary bone tumors, and they are the most commonly encountered type of malignant bone tumor (
42,
43,
44,
45 and
46). For example, 1991 projections of the number of primary bone tumors of the entire skeletal system was 2,000 compared with an estimated 1,100,000 cases of primary extraskeletal cancer for the same year (
47). Of these 1,100,000 extraskeletal tumors, 20% to 30% metastasized to bone. In 1,000 cases of biopsy-proven cancer, Abrams et al (
48) found that metastases involved bone 27% of the time. Other studies have reported similar incidences of osseous metastasis ranging between 20% and 30% (
45,
46,
49,
50 and
51). Therefore, the potential incidence of secondary bone tumors is greater than that of primary bone tumors. However, metastatic disease distal to the knee and elbow occurs relatively infrequently (
45,
52,
53).
Despite the rare occurrence of osseous metastasis to the foot and ankle, the physician must consider the possibility of an extrapedal or extraosseous primary source when a suspicious lesion is identified on plain film. The reported percentage of peripheral metastasis to the bones of the forearm, hand, leg, and foot ranges from 1.5% to 7% (
42,
54). In 1982, Zindrick et al (
55) reviewed the world literature and found 72 cases of histologically proven osseous metastasis to the foot. Other large studies have produced small numbers of pedal and ankle metastases; 4 of 41,833 cases of metastases at the Henry Ford Hospital involved the foot (
56), whereas only 10 of 75,000 osseous metastatic cases at the Mayo Clinic manifested at the foot or ankle (
50). As a caveat, these numbers may be somewhat low in that standard skeletal surveys may not include the feet, and early rectilinear (whole-body) radionuclide scans were notoriously poor in pedal imaging (
48,
51). Nevertheless, it is safe to say that metastases to the foot or ankle are rare.
Wilner defined skeletal metastatic disease as “secondary growths that have separated from the primary neoplasm and have developed as a result of the multiplication of detached transported tumor fragments” (
44). Metastatic disease is the result of a complex process. Springfield divided this process into the following steps (
57):
Cellular separation from primary tumor
Transportation to distant site (capillary or lymphatic route)
Deposition at a distant site after adherence to capillary endothelium and exit from the vessel
Establishment of supporting blood supply
Analysis of this process gives some insight into the limited involvement of metastatic disease to the foot and ankle.
Transportation of tumor emboli is accomplished by three mechanisms: direct extension, lymphatic spread, or hematogenous dissemination (
44,
45 and
46). The most important mechanism to understand is the hematogenous pathway, which involves both arterial and venous routes. Pulmonary metastases travel from a primary lung tumor through the pulmonary vein and then into the left atrium, thereby entering the arterial circulation. This is important with respect to the foot and ankle because
the majority of acrometastases originate from the lung (
44,
52,
58,
59). The venous route of dissemination is dominated by the vertebral venous system, which was first described by Batson (
60). This valveless system surrounds the spinal column and possesses interconnections with the body wall, pelvis, skull, upper extremity, and lower extremity (
44). Lacking unidirectional valves, this plexus is susceptible to intra-abdominal or caval pressure changes, and this allows for retrograde flow of caval blood into Batson venous plexus. Therefore, this represents an important vehicle for the spread of malignant disease. However, Batson venous plexus only involves the proximal half of the lower extremity and does not provide metastatic access to the foot and ankle. This limitation may partially explain the infrequent involvement of the foot and ankle in metastatic disease (
45,
46).
The location of skeletal metastases is largely influenced by the distribution and the anatomy of red marrow within the osseous system (
43,
44,
45 and
46,
61). With advancing age, red marrow is converted to yellow marrow in a distal to proximal fashion. This process begins before birth, and by 25 years of age, evidence of red marrow remains only in the following osseous structures: vertebrae, sternum, ribs, pelvis, skull, proximal shafts of femora and humeri, and possibly, to a small degree, os calcis (
61,
62). The bones containing red marrow are the preferential sites of metastatic disease and account for up to 90% of cases (
43). The remaining 10% of metastases probably originate in unconverted nests of red marrow cells surrounded by yellow marrow. Vascular anatomy predisposes the red marrow to metastasis as compared with yellow marrow. The red marrow vasculature is sinusoidal and possesses spaces or gaps between endothelial cells; these features are not seen in yellow marrow.
Thus, pooling or congestion of blood occurs in the red marrow, with an anatomic space for metastatic cellular dissemination (
45,
46).
Clinically, osseous metastases most commonly originate from a primary lung, breast, or prostate source, whereas the most common primary sites of foot and ankle metastases are the lung, kidney, and colon (
49,
50 and
51,
55,
63). Skeletal metastasis predominately affects middle-aged and elderly patients. Pain is the most common presenting clinical feature, most typically secondary to pathologic fracture. Forefoot dactylitis or nonspecific rearfoot pain may be the initial presentation of an occult extrapedal primary malignant tumor. In a report combining three separate articles, 18 of 54 cases of extrapedal neoplasia initially presented as metastases to the foot or ankle (
49,
50 and
51). Acral involvement of metastatic disease usually points to a grave prognosis because it is a reflection of widespread metastasis.
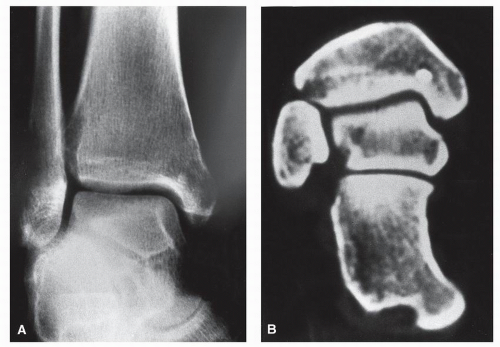
The radiographic picture can span the entire spectrum of pure lysis (renal, thyroid, lung, non-Hodgkin lymphoma, and breast) to almost pure medullary sclerosis (prostatic, Hodgkin lymphoma, occasionally breast); mixed lytic-sclerotic patterns can be seen in some cases (breast, pulmonary). Peripheral metastases usually present as permeative or “motheaten” lytic lesions. A paucity of periosteal reaction is typical (
44,
45 and
46,
49,
50 and
51,
53,
55,
63,
64). Conversely, the initial presentation may occasionally reveal a focal, lytic lesion with a
defined zone of transition between normal bone and tumor. Progressively, however, the zone of transition becomes indistinct, and the lysis involves widespread areas of one or many bones. Metastatic disease affecting the foot and ankle usually spares the joint and leaves behind a thin rim of subchondral bone; in this respect, metastatic disease may resemble chronic granulomatous infection involving the periarticular region. In this regard, one must be wary of the general differential diagnosis of the lesions that commonly involve both sides of the joint, that is, arthritis, infection, metastasis, and multiple myeloma (
65) (
Fig. 93.2).
MR Imaging: Metastatic seeding in the bone marrow generally results in either focal or diffuse areas of decreased signal intensity (SI) of marrow fat with T1W images, high SI with fatsuppressed images (e.g., STIR), and variably intermediate or high marrow SI with T2W images, depending on the particular tumor.
In several studies, MRI has actually been shown to be more sensitive than
99mTc bone scanning in detecting bone metastases (
66,
67 and
68). Although several studies have promoted the use of whole body MRI as an alternative to bone scanning in evaluating the entire skeleton for metastatic disease (
66,
67,
69), it is generally considered impractical by most—in no small part secondary to prolonged scan times and cost.
PLAIN-FILM EVALUATION—THE ESSENTIALS
The radiographic image is fundamentally a series of gray-scale tones resulting from the density or thickness differences of biologic tissues (although the atomic number of the given tissue is significant at extremely low kilovolt settings). Regions of increased density, mass, or thickness locally result in greater relative x-ray beam attenuation, which then ultimately corresponds to a locally whiter or (clearer) film image. Decreased density, mass, or thickness leads to darkening of the plain-film image. Contrast, therefore, represents the magnitude of these differences in film density (overall gray tone) across a boundary or edge. Most bone tumor imaging depends on adequate or accentuated contrast differences between the lesion and surrounding bone or soft tissue.
A basic understanding of the mechanical properties of bone is necessary to anticipate or recognize a disease and its resultant osseous responses. This is reflected in Wolff law, which states that the functional demands placed on bone control skeletal morphology. External forces create internal strains that govern local bone remodeling (
65). In response to strains generated within bone (either from external stress or from internal defect), the skeleton adapts, by bone remodeling, and attempts to provide structural support for functional demands. This structural support is radiographically manifested as trabeculae oriented perpendicular to the plane of the applied external force, thus neutralizing the external force. Trabeculae also hypertrophy or thicken in response to increased load and thereby become radiographically “whiter” or sclerotic.
Most radiographic changes accompanying osseous tumors result from the body’s response to the presence of tumor and not from the tumor itself. This response and subsequent osseous reaction occur at the cellular level. Increased tumor activity activates bone remodeling. This activation occurs secondary to local hyperemia or pressure from an enlarging mass (
18). Bone remodeling begins with osteoclastic resorption, which produces osteolysis, radiographically apparent as darkened, osteopenia, or lysis.
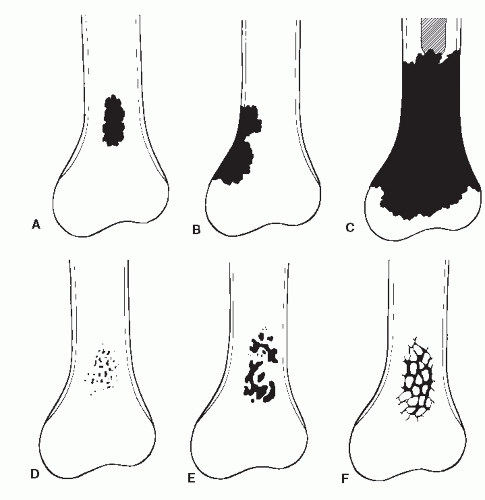
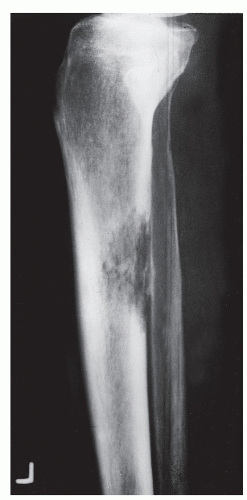
The mass of bone in this specific cross-sectional area of lysis is decreased, and increased stress is placed on bone adjacent to lysis. Bone continues to remodel around this internal defect as osseous matrix is laid down and is mineralized to resolve this stress. Thus, the repair or remodeling process is directly proportional to the biologic activity of the tumor or cohesiveness of tumor cells. Slow tumor activity allows for complete osseous remodeling, which produces a so-called benign radiographic appearance, that is, the sclerotic reactive rim surrounding a zone of geographic lysis (Ia lesion) (
Fig. 93.3). Therefore, it is also chronologically evident that the essential element in disease is considered to be the reaction of the individual. Under optimal conditions, at least 10 days must elapse before radiographic evidence of osseous cellular activity can be demonstrated (
12,
15,
25,
33). This activity may, however, be appreciated in 5 to 7 days in children.
Contrast is the essential radiographic component to visualizing a bone tumor. Even though remodeling may be occurring, it is well known that other parameters must be in accord before lytic or proliferative changes are radiographically evident. According to Madewell et al, “the perception of osteolysis on plain radiographs depends on the structure of bone (cancellous vs. cortical), the quantity of bone loss, and the amount of adjacent host bone available for contrast” (
18). Inherent bone stock in a particular region must generally be sufficient to delineate contrast at the bone-tumor interface. Underlying osteopenia, for example, can significantly diminish the contrast between the bone and tumor. Even when the underlying bone “stock” is normal, the inherent characteristics of the type of involved bone, that is, cancellous versus cortical, affect the appearance of an osseous lesion. The apparent radiographic difference between cortical and cancellous bone is directly related to the degree of “porosity” or nonmineralized tissue (
70). Cancellous bone is a richly vascularized, “porous” lattice of mineralized matrix evinced radiographically as a honeycomb of trabeculae housed in a thin, solid cortical shell. In the foot, cancellous bone comprises the tarsal bones as well as the ends of long or tubular bones. This lattice entails a much larger surface area when compared with cortical bone.
In contradistinction, cortical bone has an increased ratio of mineralized matrix to surface area and displays decreased porosity when compared with cancellous bone (
71,
72). Specifically, the nonmineralized “porous” portion of cortical bone ranges from 5% to 30%, whereas the “porosity” of cancellous bone may encompass between 30% and 90% (
70). Furthermore, there is a slight increase in the density of matrix mineralization within cortical bone. Thus, the “porosity” and, to a lesser degree, the density account for the visibly compact nature of cortical bone when compared with cancellous bone (
73).
One may therefore correctly surmise that the detection of osteolysis is easier in cortical bone than it is in cancellous bone (
15,
74). The background density of cortical bone affords greater contrast at the interface between normal bone and the tumor. This is clearly evident when one compares a lesion found in the corticodiaphysis and a lesion found in the medullary diaphysis. The cortical lesion is seen with multiple oblique views to the lesion, whereas the medullary lesion may not be detected at all with conventional radiographs (
15).
Subtle changes may be seen before overtly apparent focal lysis in areas of cancellous bone. In the metaphysis and epiphysis, a change in trabecular orientation of cancellous bone may subsequently manifest as osteolysis because this is indicative of bone remodeling (
23,
74). In bone that is not osteopenic, an area of fading trabecular density may be the precursor to frank osteolysis. Two subtle findings in the medullary canal may correlate with cellular activity: lack of clarity (haziness) at the corticomedullary junction and endosteal scalloping of cortical side of the corticomedullary junction (
13).
Recognition and evaluation of specific radiographic features are the foundation of formulating a differential diagnosis and estimating malignant potential. These can be divided into two stages. The first stage focuses on the assessment of basic anatomic characteristics of the lesion; this includes size, shape, and position within bone. However, a simple knowledge of anatomic tendencies may prove to be integral in reaching a final diagnosis. The second phase of the radiographic approach involves evaluating the osseous response to the tumor. Estimation of growth rate, appraisal of periosteal reactions, and appreciation of matrix patterns are critical steps in establishing a malignant or benign nature of a specific lesion. Although each is an indirect measure of biologic activity, collectively they can be extremely reliable when evaluated properly and placed into clinical perspective. Finally, important clinical data such as age and sex of the patient help the radiologist to generate a list of most likely diagnoses. Edeiken et al (
41) stated that, in their experience, the correct diagnosis of a specific malignant bone tumor can be made from age alone approximately 80% of the time (
Table 93.2).
Evaluation of simple characteristics such as size, shape, and position within bone initially may be overlooked or even omitted. Malignant tumors are usually larger than benign tumors. Benign tumors rarely expand to a diameter greater than 6 cm, whereas malignant tumors usually grow beyond 6 cm and often grow to more than 10 cm in diameter. Despite these general considerations, it is certainly possible for a slow-growing benign lesion to surpass 6 cm in diameter before radiographic detection. Likewise, early detection of a malignant process may occur before it reaches 6 cm in diameter. Thus, the size of a lesion is not a highly reliable sign by itself, but it can be an important consideration when reviewing the entire diagnostic picture (
1,
14,
15).
Accurate assessment of the shape of a lesion may be difficult with plain films alone. A CT scan or MRI may be needed to appreciate the morphology of a specific tumor fully. Shape is not a reliable parameter to assess the nature of a lesion. However, a constant shape without sharp changes in contour is more characteristic of a benign process (
16). In general, incipient lesions and those arising in irregular tarsal or epiphyseal bone tend to be round, whereas the oval shape predominates in those bones that display longitudinal growth through maturation (
20).
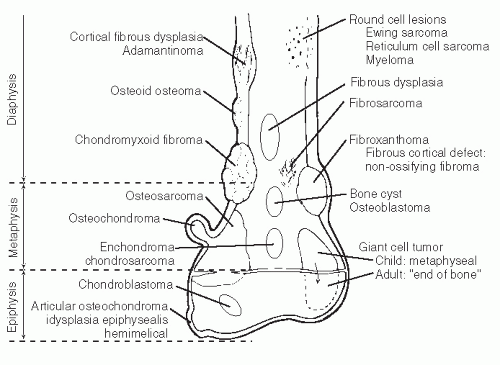
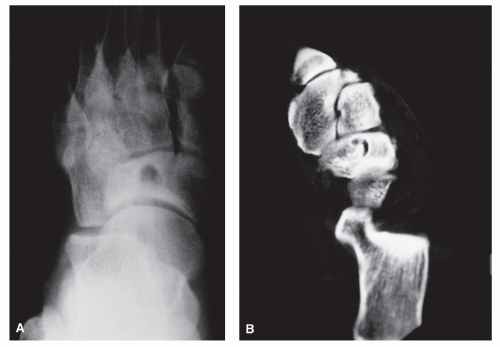
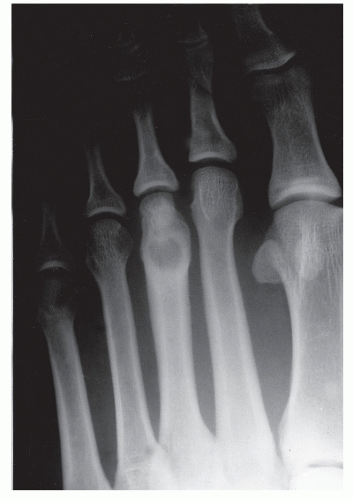
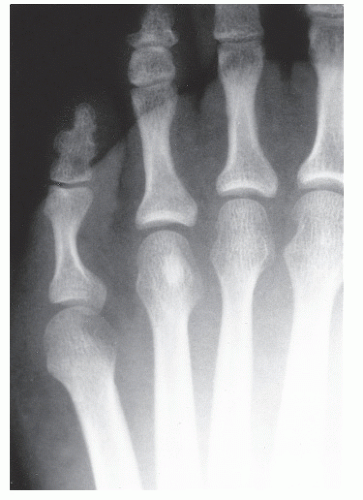
The anatomic position along the length of a bone can often give clues to the predominate cell type with respect to histogenetic lineage. Each section of bone, when considered longitudinally, has one or possibly a few predominate cell types with an inherent cellular activity. The diaphysis has an intermediate cellular activity and is composed of primarily round cells. Progressing away from the diaphysis, normal cellular activity increases. The metadiaphysis consists of primarily fibrous tissue internally, whereas externally, periosteal cellular activity predominates at the metaphyseal flare. The metaphysis possesses the highest cellular activity and is composed of osseous and cartilaginous cell types. The epiphysis demonstrates the least activity and displays an osteoclastic nature. “In general, a particular
tumor of a given cell type usually arises in that field where the homologous normal cells are most active” (
75). For example, during enchondral bone formation, osteoclasts resorb calcified cartilage. Before physeal closure, a giant cell tumor (GCT) (osteoclastoma) is most likely to occur where osteoclasts are most active: the zone of calcified cartilage. After closure of the physis, excessive bone remodeling takes place at the site of the previous physis. The first step in bone remodeling is bone resorption or osteoclastic activity; thus, a GCT may be seen at the metaphysis, the metaepiphysis, or the epiphysis once the physis is closed (
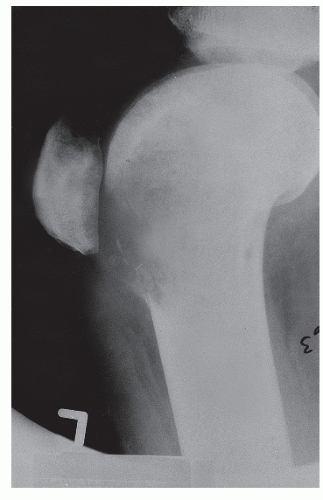
75) (
Fig. 93.4).
GRADING OR GROWTH RATE MARGINS
The manner in which bone loss presents on plain-film radiographs over time correlates to the rate of growth of the specific lesion. Bony destruction is analyzed and placed into five separate
grades: Ia, Ib, Ic, II, and III. These number grades are not exactly analogous to the aforementioned numeric assignments to the type of destruction. Rather, they are meant
to correlate with a specific lesion’s probability of being benign or malignant. Grade Ia is latent or the most benign, whereas grade III has the most malignant characteristics. The analysis is based primarily on the pattern of destruction—geographic, moth-eaten, or permeative, but other factors such as the presence of a sclerotic rim surrounding the lesion, the degree of cortical integrity, the presence of a soft tissue mass, and the appearance of an expanding cortical shell also aid in placing a lesion into an appropriate grade.
Space-occupying lesions of bone become radiographically evident as the contrast across their margin increases. Intact trabeculae of cancellous bone adjacent to the edge of the lesion heighten contrast. Imaging of lesions that are solely intramedullary within the diaphysis therefore becomes problematic because of the lack of marginal cancellous bone (
18,
80). Indeed, proliferative changes such as a rim of sclerosis or increases in trabecular texture are seen earlier and more easily in the metaphyseal and epiphyseal cancellous bone. However, the proliferative response of the periosteum is appreciated better in the cortical bone of the diaphysis. The final grade is ultimately a representation of the cellular activity for a specific instance in time. Sequential grades of the same lesion over time will give insight into the growth rate of that particular tumor. The role of the initial grade is to direct the subsequent management, that is, serial radiographic evaluation or surgical biopsy for histologic analysis.
The margin depicts the interface between the tumor and the adjacent host bone. In benign processes, the margin is typically distinct. Slow-growing processes can produce complete osteolysis (no remaining trabeculae surrounded by tumor) within a given area of bone with narrow zones of transition between tumor and host bone. The end result is a geographic area of osteolysis with a well-demarcated peripheral edge. If growth is slow enough, proliferative adaptation of the surrounding bone will take place and will produce a rind or rim of sclerosis. This rind of sclerosis, the most reliable sign of indolence, is benign to the ninety-eighth percentile (
13). As the margin becomes less distinct, one sees a broader zone of transition. An imperceptible margin with diffuse, infiltrative destruction points toward a fast-growing, malignant process. The five grades employed in radiologic growth rate assessment are described in the following paragraphs.
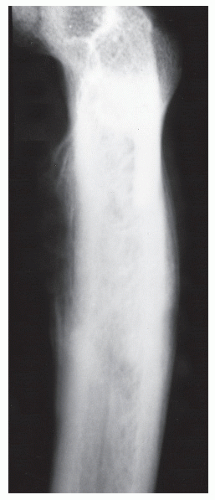
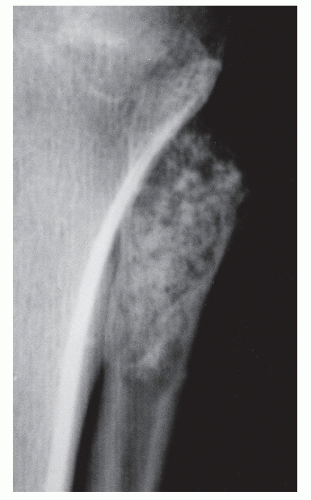
GRADE IA: GEOGRAPHIC DESTRUCTION WITH SCLEROTIC MARGIN
This is considered the slowest grade of growth rate and is most likely a benign process. The radiographic margin correlates with the actual histologic edge of the lesion. In neoplastic diseases, the outer edge of sclerosis is well defined and the inner border of sclerosis is poorly defined or “hazy,” whereas chronic infectious or inflammatory Ia lesions have fading or poorly defined outer sclerotic margins and sharply defined inner sclerotic aspects. The differential diagnosis includes bone cyst, fibroxanthoma, fibrous dysplasia, chondroblastoma, chondromyxoid fibroma (CMF), enchondroma, osteoblastoma, Brodie abscess, and eosinophilic granuloma (
Fig. 93.8).
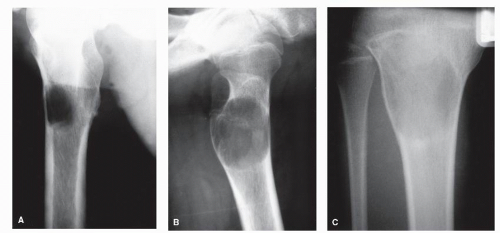
GRADE IB: GEOGRAPHIC DESTRUCTION WITHOUT SCLEROTIC MARGIN BUT WITH A NARROW (WELL-DEFINED) ZONE OF TRANSITION
Grade Ib growth rate suggests only slightly faster growth rate than Ia, but it is still a slow-growing lesion that is most likely benign. The radiographic margin correlates with the actual histologic edge of the lesion. Intramedullary Ib diaphyseal tumors, without significant cortical involvement, may appear almost invisible inasmuch as there is no cancellous bone to define their edge. In this case, only internal cortical scalloping may then suggest the presence of this lesion (
18). GCTs are prototypical Ib lesions. The differential diagnosis of Ib lesions generally otherwise includes the previous Ia lesions (
Fig. 93.9).
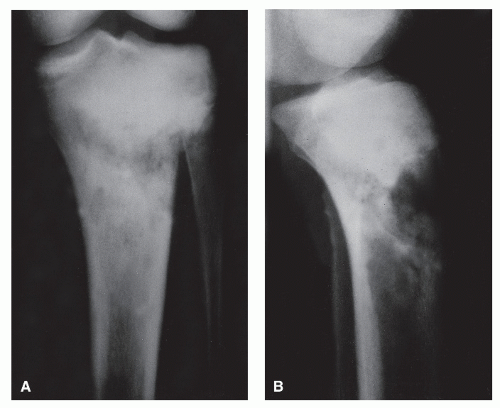
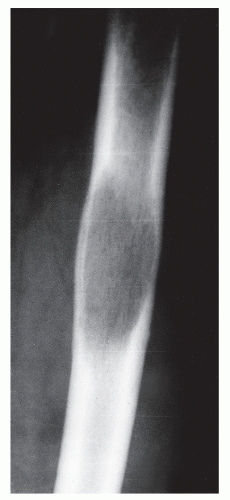
GRADE IC: GEOGRAPHIC DESTRUCTION WITH AN ILL-DEFINED MARGIN
Grade Ic lesions are more aggressive than Ib lesions and should be thought of as equivocal lesions, that is, not clearly benign. In these lesions, the destructive process probably extends beyond the radiographic margin (
10,
21) (
Fig. 93.10).
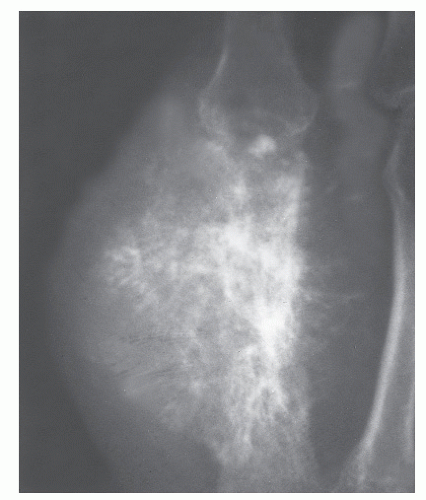
GRADE II: COMBINATION OR CHANGING PATTERN WITHIN A SINGLE LESION
These lesions are often admixtures of geographic lysis and moth-eaten or permeative destruction. This pattern suggests a much more aggressive process than grade I lesions, and cortical integrity is often suspect. Grade II growth rates may be seen in association with malignancies such as fibrosarcoma and aggressive GCTs, osteomyelitis, and histiocytosis (
10,
21) (
Fig. 93.11).
GRADE III: OVERT MOTH-EATEN (GRADE IIIA) OR PERMEATIVE (GRADE IIIB) DESTRUCTION
These are the most aggressive lesions. They have no suggestion of a preexisting geographic lesion. Cortical integrity is commonly violated with an associated soft tissue mass in this grade.
PERIOSTEAL REACTIONS
Periosteal reactions are a general indicator of the biologic activity of a bone lesion (
25). The radiographic data obtained in the evaluation of a periosteal reaction associated with a bone tumor can provide important information on whether the lesion is aggressive or not. Nevertheless, even though periosteal reactions are a reflection of the biologic activity of a bone lesion, it is generally a mistake to think that given morphologic features of a periosteal reaction are specific, pathognomonic, or diagnostic of any particular bone tumor. As an added caveat, even the more aggressive periosteal patterns have been occasionally noted with benign bone tumors. Therefore, it is accurate to think of classifying the periosteal reaction as a “puzzle piece” to be combined with other data such as type of destruction, margination, and growth rate assessment and matrix determination.
Much of the discussion in the following sections on the radiographic evaluation of periosteal reactions and matrix formation has been condensed from articles as well as our own experience in applying these classification principles to the radiographic analysis of periosteal reactions (
19,
25).
The periosteum is an overlying covering or sheath around bones that plays a role in bone turnover throughout development and life. It is specifically composed of an outer fibrous layer and an inner cellular cambium layer. The periosteum is joined to the underlying cortex by perpendicular collagenous strands known as Sharpey fibers (
10,
22,
81). The periosteum around the normal adult long bone is relatively dormant and mainly fibrous, with minimal cellularity. However, during periods of rapid normal growth, response to injury, or some local or central pathologic stimuli, the periosteum can become thick, and the two layers can be distinctly separate. Nevertheless, Ragsdale et al suggested that the delineation of two distinct layers of the active periosteum is a convention that can be misleading because it obscures the probable likelihood that the plane between these layers is a transitional zone; the fibrous layer can be replenished from the surrounding parosteal soft tissues such as fascia, fat, and muscle (
19,
25). The earliest phase in this process is the progressive modulation of fibroblasts to active “preosteoblasts.” Ultimately, the transition of these cells to osteoid-secreting cells is made within the outer fibrous layer. Often, new bone formation around the cortex is noted in this fashion by parosteal soft tissue structures without the formation of the traditional “dual-layered” interface or periosteum (
19,
25).
Once the periosteum has been activated to produce new bone by some stimulus, a lag occurs in the time it takes for the appearance of mineralization on the radiograph. Even with vigorous periosteal activity, it can take from 10 to 21 days after the initiating stimulus for periosteal mineralization to be appreciated. Periosteal reactions become apparent earlier in younger people. The appearance of a periosteal reaction in a pathologic process is variable; it is rarely noted with tumors such as chondrosarcomas and fibrosarcomas, whereas it is typically present with Ewing sarcoma, osteosarcoma, and solitary metastasis (especially osteoblastic) (
10,
82). The periosteum is commonly elevated in infectious processes such as osteomyelitis and subperiosteal abscess by pus, by dilated periosteal vessels or edema, by passive hyperemia (
10,
19,
83), or by tumor or hemorrhage (
1,
10,
17,
21,
81).
The nomenclature of lesions affecting the bony surface can be confusing. In general, the lesions of juxtacortical origin can be divided into cortical, subperiosteal, periosteal, parosteal, and paraosseous lesions (
84).
Subperiosteal lesions separate the periosteum from the cortex and result in subperiosteal new bone formation. Often, these bony lesions leave the underlying cortex grossly intact, and they have entered the subperiosteal space by cortical “percolation” through Volkmann canals and haversian systems or, less commonly, by vascular penetration (diaphyseal vessels) (
19).
Periosteal lesions originate from the deep layer of the periosteum and are usually firmly attached to the cortex. As this type of tumor (e.g., periosteal chondroma) enlarges, the periosteum may locally be elevated. The “sunburst” periosteal reaction is probably an aggressive form of periosteal lesion (e.g., osteosarcoma) (
84).
Parosteal lesions originate from the outer fibrous layer of the periosteum, whereas
paraosseous lesions originate from completely outside the periosteum and display a soft tissue cleavage plane separating the lesion from the periosteum and cortex (
84).
With respect to the radiographic analysis of periosteal reactions of bone tumors, paradoxically, it is often the case that “nothing is so bad as something that does not look so bad.” For example, imposingly thick, homogeneous periosteal reactions often accompany slow-growing, benign lesions, whereas more
aggressive lesions have finely layered, wispy, spiculated, porous, or lace-like patterns. In the latter instance, lace-like periosteal reactions are particularly worrisome, inasmuch as they are easily confused with inflammatory soft tissue swelling on plain-film radiographs.
Morphologically, periosteal reactions are best evaluated with the classification system outlined by Ragsdale et al (
19), whereby they are divided into three general categories: continuous, interrupted, and complex (
19,
25). Continuous periosteal reactions are then further subdivided based on whether the underlying cortex is intact or has been resorbed in association with the appearance of ballooning-type expansion (
19,
25).
CONTINUOUS PERIOSTEAL REACTIONS WITH THE UNDERLYING CORTEX DESTROYED
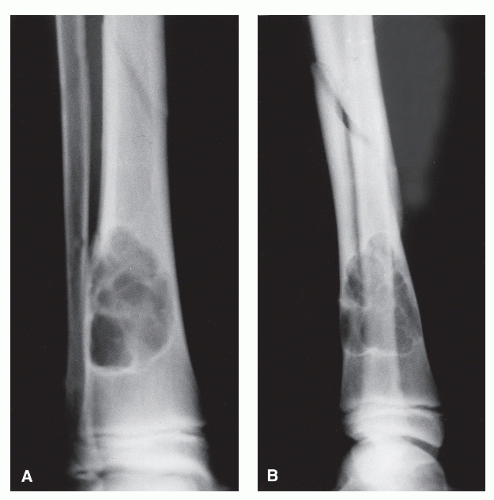
Bone is a rigid structure that has virtually no capacity to expand or “balloon.” For bone to display these structural alterations, significant endosteal resorption of the original cortex must have occurred at a rate greater than outer periosteal new bony “shell” formation around the bone tumor. Although this is typical of many geographic lesions (especially those arising within small or narrow tubular bones, e.g., phalanges, metatarsals and fibula), any sudden expansion of an existing benign bone lesion should be viewed as an aggressive change in its biologic growth rate and therefore may represent malignant degeneration. Uniform replacement of the original cortex usually results in smooth shell expansion. This is typical of tumors such as unicameral bone cysts, intraosseous lipomas, fibrous dysplasia, and chondroblastomas. Lobulated or trabeculated new periosteal cortical shells contain ridges and furrows that are apparent on the radiographs. These sclerotic “weaves,” which appear to be regularly or irregularly interspersed throughout the tumor, probably represent normal cortex that has remained relatively intact around areas of asymmetric or irregular tumor growth. Lobulated shells may develop trabeculated patterns. Although tumors such as GCTs, desmoplastic fibromas, and aneurysmal bone cysts (ABCs) may present with smooth cortical shell, they most typically are characterized by trabeculated cortical expansion. The trabeculation is fine in the case of ABCs and GCTs. Other tumors such as enchondromas, nonossifying fibromas, CMFs, and slow-growing malignant tumors may also present with trabeculations.
CONTINUOUS PERIOSTEAL REACTIONS WITH THE UNDERLYING CORTEX INTACT
Most of these patterns arise secondary to the subperiosteal presence of some pathologic process whereby the underlying cortex persists.
SINGLE LAMELLAR AND SOLID REACTIONS
Initially, a single layer of periosteal new bone may be apparent, with the underlying bony cortex grossly intact. At this time, the underlying lesion may or may not be aggressive, although most typically it is benign in nature. If a single layer homogenously thickens beyond 1.0 mm and remains continuous, it is then most appropriately labeled as a solid periosteal reaction. It must be noted that these reactions are nonspecific and may be seen locally in many benign processes such as infection, medullary infarction, consistent (nonepisodic) irradiation (
85), trauma, as well as with malignant processes (
1,
19,
85). Solid periosteal reactions can also arise from the thickening of a single layer or by slow formation of an overlying new layer of bone that binds with the original layer of bone. In either case, s
olid periosteal reactions are generally held to be the radiologic hallmarks of benign processes (
41), and they are typically seen in conditions such as osteomyelitis, eosinophilic granuloma, bone abscess, myositis ossificans, healing fractures, and subperiosteal hematoma. The tendency is for the limited resolution of the plain-film radiograph to obscure the true nature of finely spiculated or lamellar periosteal reaction patterns and to produce an almost solid-appearing reaction.
MULTILAMELLAR REACTIONS
Unlike solid periosteal reaction formation, the single lamellar periosteal reaction may progress with the formation of a continuous multilamellar or “onion-skin” periosteal reaction composed of multiple layers of ossification clearly separated by radiolucent spaces of variable thickness. This periosteal reaction is thought to arise from the episodic formation of concentric “planes of ossification beyond the cortex,” probably the result of cyclic variation in subperiosteal tumor growth, that is, with repeated phases of rapid periosteal elevation. Lamellated periosteal patterns may be seen in both benign and malignant processes. Rapid or aggressive processes have a tendency to display thinner ossific planes and wider spaces and vice versa (
10,
19). The tendency is to overplay the association of multilamellar periosteal reactions with malignant processes. Although these reactions are commonly noted in association with Ewing sarcoma, they clearly are not pathognomonic for this tumor. In our own clinic, multilamellar periosteal reactions have occasionally been noted in association with such benign processes as osteomyelitis, histiocytosis, ABCs, and hypertrophic pulmonary osteoarthropathy, as well as with other malignant bone tumors such as osteogenic sarcoma and metastases (
10,
19) (
Fig. 93.12).
PARALLEL, SPICULATED PERIOSTEAL REACTIONS
These patterns have also been described as “hair on end” patterns. Generally associated with rapidly aggressive processes (
10,
19,
22), these periosteal patterns can be seen in malignant tumors such as Ewing sarcoma and occasionally osteosarcoma. However, these reactions are also seen in marrow disorders such as sickle cell anemia and thalassemia, as well as in certain inflammatory disorders such as syphilis and myositis (
1,
10,
16,
19,
25). Two-dimensional radiographic projections obscure the finding that these are actually ossific walls of a honeycomb structure in the subperiosteal space. This is the result of osteoblasts that become oriented midway between vertically oriented periosteal blood vessels extending from the cortex to the periosteum (
19,
25).
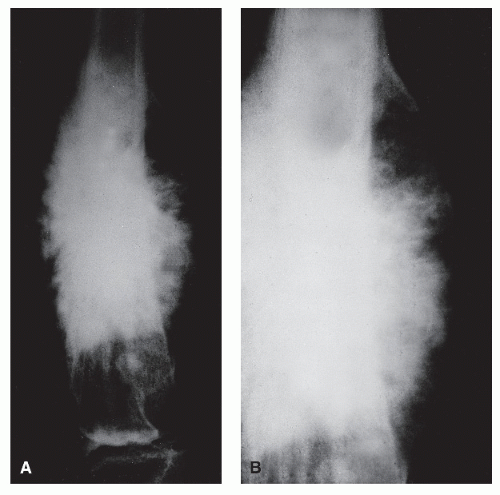
DIVERGENT, SPICULATED (SUNBURST) PERIOSTEAL REACTIONS
As the name implies, long perpendicular spicules of delicate subperiosteal new bone formation appear to emanate from a common center and diverge as they extend from the cortex to the periosteum. Although it is considered an aggressive pattern, the underlying cortex generally persists. Most suggestive of osteogenic sarcoma, sunburst periosteal reactions are not
pathognomonic for the underlying presence of this tumor because they have been occasionally noted in osteoblastic metastases from breast and prostatic carcinoma, actinomycotic osteomyelitis, and hemangioma (
19,
22). However, as Nelson pointed out, this association is extremely rare and is hardly ever seen in pyogenic osteomyelitis. As a variety of spiculated pattern, it is best classified as a complex type of reaction (
Fig. 93.13).
INTERRUPTED PERIOSTEAL REACTIONS
Any one of the aforementioned periosteal reactive patterns may be resorbed by a tumor and may thereby produce the appearance of an interrupted periosteal reaction. This strongly suggests that the bone tumor has become extraosseous, that is, in the surrounding soft tissues. Furthermore, when the periosteal reaction has been lysed or resorbed centrally or at its middle to form an interrupted “cuff” of new bone on either side of the primary focus, sarcoma is strongly suggested (
19,
86). A clearly defined extraosseous soft tissue mass associated with an underlying bony lesion is almost always caused by a neoplasm and is rarely the result of a bone infection (
22).
BUTTRESS REACTIONS
Buttress reactions (periosteal “buttressing”) present as if they are interruptions of solid periosteal new bone formation and as such appear as solid triangular wedges of periosteal bone that form (or remain) at the lateral extraosseous margins of slowly enlarging bone lesions. These lesions are commonly associated with geographic destruction with a shell-type periosteal reaction or are otherwise of periosteal or parosteal origin. Solid periosteal buttresses are typically found in lesions such as CMF and juxtacortical chondroma, but not exclusively benign lesions. Sarcomas
have presented with radiographic findings of what appears to be a buttress periosteal reaction. Sometimes, magnification radiography or histologic examination reveals the appearance of an interrupted fine, multilaminate periosteal reaction forming the buttress. Occasionally, buttress reactions form by the resorption of a preexisting solid periosteal reaction and should be considered a sign of aggressive change in a previously benign process (
19). An exception may be osteomyelitic cloaca formation.
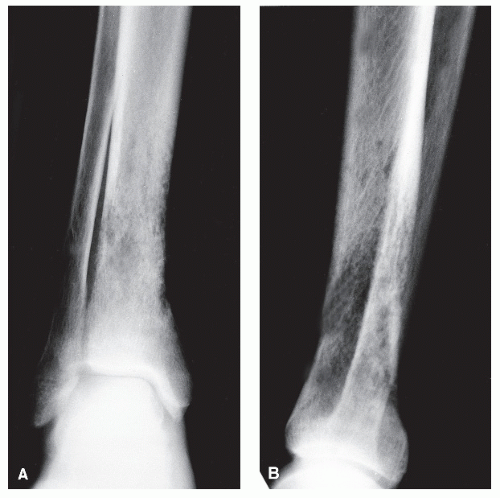
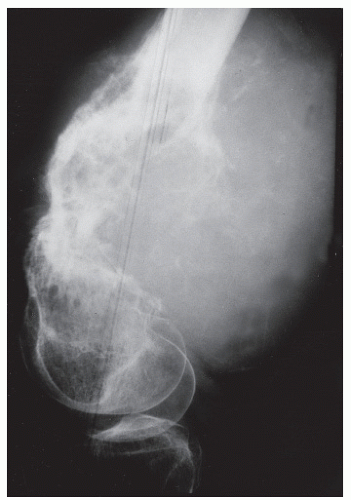
CODMAN ANGLE
These features are angular zones of periosteal bony formation at the edge of a lesion (
3,
17). In contradistinction to triangular buttress reactions, Codman angles are best referred to as angles because the base of the lesion is radiolucent (and only two sides of a triangle are noted). Although not pathognomonic for malignancy, the presence of a Codman angle generally suggests the presence of an aggressive malignant lesion that has broken out of the bone and into the surrounding soft tissue. This is especially true when multiple Codman triangles become radiographically manifest (
10,
87). Interrupted lamellated patterns may appear as buttress patterns when the layers are numerous and thin, and they are generally aggressive bone tumors. Eosinophilic granuloma in children is an exception. Interrupted spiculated periosteal reactions are most often associated with aggressive tumors of medullary origin, such as Ewing sarcoma, as well as with surface parosteal osteogenic sarcomas (
Fig. 93.14).
COMPLEX PERIOSTEAL REACTIONS
Complex periosteal reactions are basically admixtures or variations of the aforementioned types. Generally, the more complex the periosteal reaction, the more aggressive it is likely to be. As stated before, the well-known sunburst divergent spiculated pattern is commonly a sign of malignant tumor osteoid production and not just a sign of periosteal reactivity. So-called lace-like periosteal reactions (resembling millinery or handkerchief lace), which have been associated with aggressive tumors such as Ewing sarcoma in children, are probably best included in this category. These reactions are of particular concern because their radiographic ossification pattern can be so fine as to mimic inflammatory soft tissue swelling on plain-film radiographs and may thereby suggest a lesion of lesser activity.