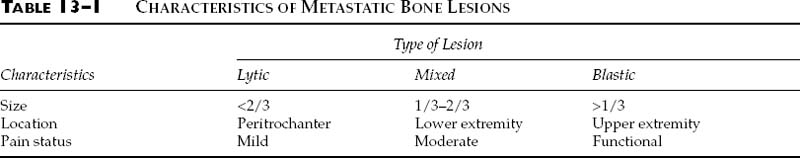
Chapter 13 The human skeleton is perfectly adapted to allow human motion and numerous activities. Bone also has the unique ability to repair without scar formation. The healthy skeleton will rarely fracture during normal physiologic motion, but fracture may occur when large loads are applied directly to the skeleton by events such as falls from heights, motor vehicular accidents, gunshot injuries, twisting injuries, or the contraction of large muscle groups. In addition, however, there are a number of conditions that may weaken the skeleton and predispose it to fracture with normal physiologic loading. These conditions cause either a focal loss of mineral in the skeleton, as in metastatic bone disease, or a very diffuse and symmetric loss, as in osteoporosis. Metastatic bone disease is common. Each year in the United States there are approximately 1.3 million new cancer cases, and many of these patients will develop metastatic bone disease.1 The most common carcinomas that metastasize are those of the breast, lung, prostate, and kidney. These metastases typically occur in multiple bones and are usually distributed to the spine, ribs, pelvis, and long bones. There are three predominant patterns of skeletal involvement: (1) purely lytic bone destruction, (2) a mixed pattern of bone destruction and bone formation, and (3) purely blastic disease without bone lysis. These patterns are determined by the interaction between the metastatic cells and the host bone. Pure lysis occurs when the bone is resorbed and there is no concomitant bone formation; a mixed pattern occurs when there is both bone destruction and formation; and a purely sclerotic pattern occurs when there is extensive bone formation without a loss of mineral. Patients with metastatic bone disease face a number of clinical problems, including pain, inability to ambulate, and pathologic fractures. For the cancer patient, uncontrolled bone metastases severely compromise the remaining quality of life (e.g., patients may lose the ability to ambulate and care for themselves). In the cancer patient, an untreated fracture results in severe pain; such patients often lose the will to live and spend their remaining days in despair. Physicians play a major role in the treatment of patients with metastatic bone disease. The primary goals of treatment are to control pain, prevent pathologic fractures, and maintain patient independence. This chapter will address the effect of metastases on bone, the indications for prophylactic fixation, and the principles of surgical management. The presence of a tumor in bone changes the mechanical properties of that bone. Existing data suggest that lytic lesions decrease not only the stiffness but also the strength of the bone in bending.2 In contrast, blastic lesions decrease only the bone’s stiffness, not its strength.2 The location of the lesion within the medullary canal also changes the strength of the bone. If the medullary defect is located within the center of the diaphysis, the strength reduction for a 50% loss in cross-sectional area is 60%. However, with the same amount of bone loss for an eccentrically located lesion, the strength reduction is greater than 90%.3 Pathologic lesions in long bones also decrease the strength of the bone in torsion. Experiments on animal models have shown progressive reduction in torsional strength with increasing size of the cortical defect.4 The length of the lesion within the medullary canal does not substantially alter the bending properties; however, it does reduce the torsional strength.4 Biomechanical studies have shown that a lesion involving one third of the width of the bone reduces its torsional strength by 60%.5 One of the most common radiographic criteria for operative management of a lesion is its size.6–12 It must be noted that plain radiographs tend to underestimate the amount of bone loss, and accurate determination of bone loss becomes more difficult when the lesion does not have clear boundaries. In a report on 19 pathologic femoral fractures secondary to breast metastases, Snell and Beals7 noted that 58% of those fractures were predictable when a lesion of 2.5 cm in diameter involved the femoral cortex or was painful, regardless of the bony location. Parrish and Murray13 reported on 104 pathologic fractures secondary to metastases and recommended prophylactic fixation in patients with increasing pain and loss of one third of the diameter of the bone. In 1973, Fidler9 evaluated 19 pathologic fractures in long bones and analyzed the size of the lesion and level of pain before fracture. He recommended using prophylactic fixation if a lesion involved more than 50% of the diameter of the bone and also advocated not using pain as an indication for prophylactic fixation. Harrington10 recommended prophylactic fixation for lesions 2.5 cm or larger, lytic destruction of 50% or more of the cortex of a long bone, or persistent pain with weight bearing despite local radiotherapy. Fidler14 later evaluated 66 patients with 100 consecutive metastases in long bones, measuring the size of the defect and determining the risk of pathologic fracture. He concluded that fractures were unlikely when less than 50% of the cortex was destroyed (2.3%), likely when 50% of the cortex was destroyed (60%), and most likely when more than 75% of the cortex was destroyed (80%). However, most of the above indications cited in these studies were based on retrospective reviews of femoral lesions, and the specific criteria were based on general impressions rather than on precise biomechanical modeling or randomized prospective studies. Mirels15 developed a classification system that incorporated the pattern of bone destruction, size and location of lesion, and degree of pain. He reviewed the files and roentgenograms of 38 patients with 78 metastatic lesions of long bones. In most cases (53/78, 68%), the etiology was metastatic breast cancer. He analyzed the risk factors for pathologic fractures by evaluating four variables: the patient’s pain and the location, type, and size of the lesion (Table 13–1). In this system, each variable is scored from 1 to 3, with a maximum possible score of 12. A patient with a score of ≤7 has a low risk of fracture, whereas a score of ≥9 indicates a high risk. A score of 8 is suggestive, indicating a fracture risk of 15% with a false-positive rate of 6%. A score >9 is diagnostic for prophylactic fixation (Table 13–1). The most limiting aspect of this system is the subjectivity of grading the variables: characterizing the size of an individual lesion is difficult, and assessing pain is very subjective for both the patient and examiner. Orthopaedic surgeons frequently evaluate patients with metastatic lesions and must make a determination of the risk of pathologic fracture. The amount of bone destruction is measured on both anteroposterior and lateral plain radiographs, and the pattern of metastatic bone involvement is graded as purely lytic, mixed lytic and blastic, or purely blastic (see above). Patients are also asked whether their pain occurs with rest or with weight bearing. Patients with metastatic bone disease can lead fairly normal lives if the destruction of the skeleton can be controlled and limited, especially in terms of impending fracture (defined as a state in which the bone has been weakened to such an extent that normal physiologic loading will result in fracture). There are nonoperative and surgical modalities for accomplishing this goal. There are several relative contraindications to managing metastatic bone disease surgically: (1) a patient who has a very short life expectancy (less than 2 to 4 weeks); (2) a patient who is too ill to survive a surgical procedure; (3) a patient with neutropenia; and (4) a patient with a low absolute white blood count and, thus, a high risk of postoperative infection, for whom surgery should be delayed until the immune system recovers. Such patients are best managed nonoperatively with external beam irradiation to control pain and limit disease progression. If a patient has two or more of the following general criteria, we recommend prophylactic surgery: (1) >50% cortical bone destruction in a long bone; (2) long lytic lesions (length more than two or three times the bone’s diameter); (3) purely lytic pattern of bone destruction; (4) weight-bearing pain; or (5) pain after irradiation. The high stress areas at particular risk of fracture are the subtrochanteric region of the femur, the femoral diaphysis, the humeral diaphysis, the femoral neck, and the intertrochanteric hip area. Activity-related pain in these regions is often a harbinger of fracture. Surgical intervention to strengthen the bone, prevent fracture, and allow full and painless weight bearing can be performed via a variety of advanced techniques. The method chosen should be durable to minimize the risk of fixation failure. Options include intramedullary rods, plates and screws, and prosthetic devices, and the choice is based on the amount of bone destruction and the location of the lesion. Acrylic (methylmethacrylate) cement is a useful adjunct to fixation because it hardens quickly and provides additional strength. Surgical intervention is usually followed by irradiation to prevent progression of the bone destruction. Intramedullary nails, the most common internal fixation devices for patients with metastatic bone disease, are well suited to providing rigid fixation for several reasons (Fig. 13–1
BONE FIXATION IN PATIENTS
WITH BONE TUMORS
EFFECT OF METASTASES ON BONE
INDICATIONS FOR PROPHYLACTIC INTERVENTION
NONOPERATIVE MANAGEMENT
SURGICAL MANAGEMENT
Intramedullary Nails
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree