Chapter 22 Molecular biology of neoplasia
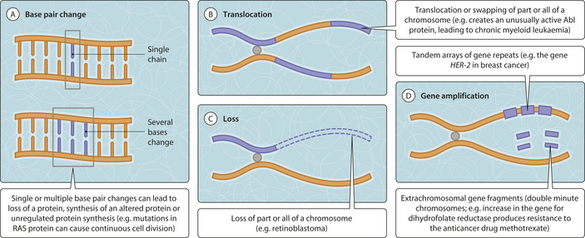
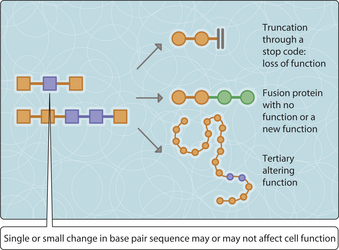
The development of neoplasms is associated with the gradual accumulation of genetic mutations within the neoplastic cells. Some mutations have no discernable effect while others can lead to serious neoplastic or non-neoplastic diseases. Changes to the genetic material may be substitution or loss of a single DNA nucleotide, a small series of nucleotides or part or the whole of a chromosome (Fig. 3.22.1). Even a single nucleotide substitution can result in gross damage (Fig. 3.22.2). Sometimes a germline (i.e. inherited) genetic mutation is already present within the non-neoplastic cells and this can accelerate the process of neoplastic transformation (Ch. 33). Study of the mutations occurring within neoplastic cell populations can provide an insight into neoplastic transformation and the development into malignancy. Mutations important in the initiation and progression of neoplasia tend to occur within genes whose products are involved in the regulation of cellular growth and differentiation (i.e. the process of maturation from primitive but multipotential stem cells to fully functional and specialized cells).
Genetic mutations and neoplasia
Tumour suppressor gene describes a gene whose normal function is essential for the prevention of neoplasia development (Table 3.22.1). Although each normal cell contains two copies of each of these genes, only one correctly functioning gene is required to prevent neoplasia development. Certain inherited cancer syndromes are characterized by the inheritance of one non-functioning copy of a particular tumour suppressor gene, with mutation of the second copy occurring at the site of tumour development. Knudson was the first to describe this process as the ‘two-hit hypothesis’, initially in the context of retinoblastoma (a malignant tumour of the retina). Patients with a family history of retinoblastoma (and therefore inheriting a mutation within one copy of the retinoblastoma tumour suppressor gene) developed tumours at a younger age, and more commonly bilaterally, than those without an apparent inherited predisposition. This observation was consistent with the hypothesis that inheritance of one mutated gene was associated with a greater risk of neoplasia since prevention of neoplasia was dependent upon the correct functioning of a single copy of the gene.
Table 3.22.1 Tumour suppressor genes
| Gene | Function | Clinical manifestationa |
|---|---|---|
| p53 | Prevention of cells containing genetic mutations from progressing to mitosis; promotion of apoptosis of these affected cells | Li-Fraumeni syndrome (multiple cancers, especially carcinomas and sarcomas) |
| hMLH-1, hMSH-2 | Repair of genetic mutations | Hereditary non-polyposis colorectal cancer syndrome (especially of proximal colon; endometrial cancer) |
| RB1 | Regulation of the cell cycle | Retinoblastoma, osteosarcoma |
| APC | Uncertain | Familial adenomatous polyposis |
| BRCA-1 | Growth control | Breast and ovarian cancer |
| BRCA-2 | Uncertain | Breast cancer |
a Associated with inheritance of germline mutation; however, somatic mutations within some of these genes may also be found in tumour tissue in sporadically occurring cancers.
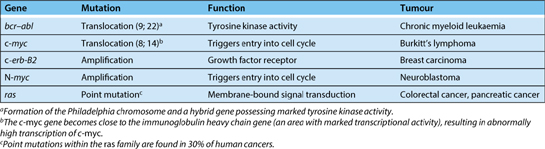
Genes whose normal function is involved with the control of cellular growth or proliferation are known as proto-oncogenes. Mutations that result in the abnormal or uncontrolled activation of proto-oncogenes may lead to neoplasia and in this situation these genes are termed oncogenes. Oncogenes contribute to the development of neoplasia through failure of the normal mechanisms regulating cellular growth and proliferation (Table 3.22.2).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree