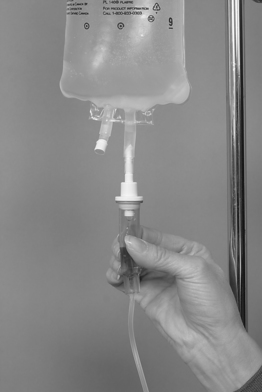
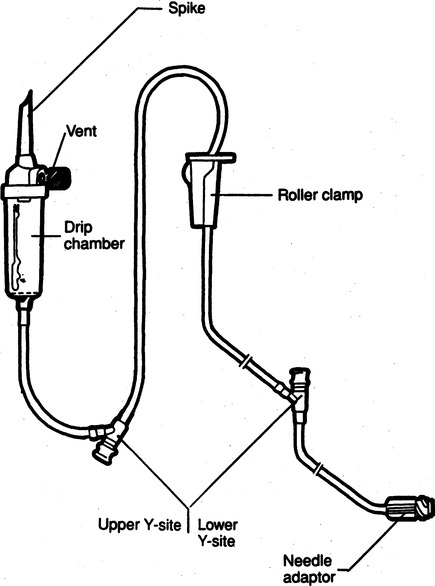
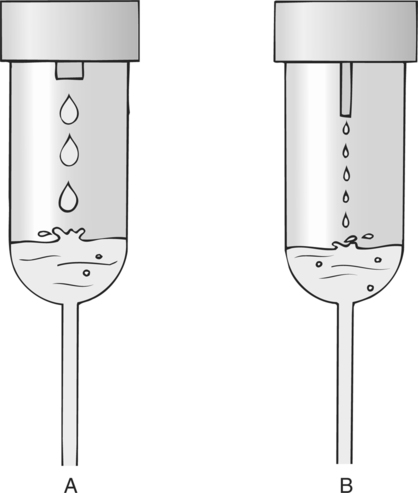
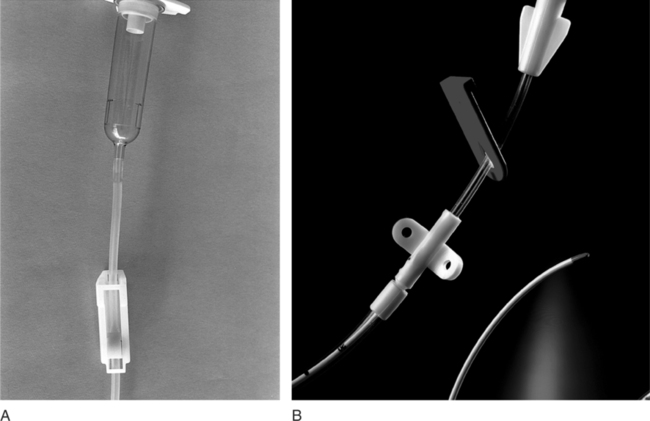
Chapter 4 Upon successful completion of this chapter, the student will be able to: • Identify and define the equipment needed for initiation of intravenous therapy. • Identify the types of containers holding intravenous infusion fluids. • Identify the types of intravenous administration sets and the uses of each type as primary and secondary lines. • Describe the differences in peripheral or central infusion devices. • Describe the advantages and disadvantages of needles, over-the-needle catheters, and scalp vein or winged needles and the selection for infusion therapy based on patient needs. • Discuss the basic types of intravenous solutions and the primary uses for each. • Describe the types of devices used to regulate flow rates of intravenous infusions. • Decide the adjunct supplies, such as those for dressings, that are needed for safe infusions for patients. device in the primary tubing that prevents the backflow of secondary infusates into the primary line. amount of medication given rapidly intravenously that is used to provide a rapid response to the medication or to give a dose to raise the level of the drug in blood. sheath used to infuse fluids into the vein. hollow flexible tube that is inserted into a vessel or cavity to instill fluids. suspension in which small particles are dispersed through the liquid. solution in which the substances are dissolved in the fluid; a fluid that has the ability to diffuse through a semipermeable membrane. elongated, enlarged rigid section located at the top of the tubing; holds fluids for administration between the supply container and the tubing. number of drops needed to deliver 1 mL of fluid. opening in the top of the drip chamber that determines the size and shape of the drop. speed at which IV fluids are regulated for administration. solution that causes a flow of water out of the cell across its semipermeable membrane into the vascular system. solution that causes the flow of water into the cell across its semipermeable membrane from the vascular system. parenteral fluid that is slowly introduced intravenously over a specific period. apparatus designed to deliver a predetermined amount of IV solution or drug through an IV injection over a certain period of time. accesses located along primary administration tubing that are used for the administration of secondary infusates. solution in which the body tissues can be bathed without the transfer of fluids across the semipermeable membrane of a cell; IV solution with the same tonicity of body fluids. set of short tubing that has a standard drop factor of 10 to 20 drops/mL that is added to the injection port of the primary administration set; secondary line for administering infusates over a short time. tubing that supplies large drops of fluids, such as 8 to 20 drops/mL. tubing that supplies small drops of fluids, such as 50-60 drops/mL. blunt-tipped plastic insertion device that is inserted into an injection port for the secondary administration of infusates without the use of a needle; the system opens the port and reseals the port on removal. infusion set that uses a needle as a stylet and leaves the catheter found over the needle in place for the infusion. peripherally inserted central catheter (PICC) long IV access device made of a soft, flexible material that is inserted peripherally and threaded into the superior vena cava. scalp vein or butterfly needle infusion needle that has plastic holders on each side of the needle hub to help hold the needle in place during infusion. sharply tipped plastic end of the drip chamber that is inserted into the infusate container to allow the flow of the infusate from the storage container into the tubing. needle or guide that is found within a catheter to allow penetration of the vein and is then removed, leaving the catheter in place. catheter that is 8 to 36 inches long and lies within a plastic or metal encasement for the insertion into a vein for IV therapy; the encasement is removed after insertion and the catheter is left in place. Most IV fluids today are in closed containers made of flexible plastic. Closed containers are those that do not require venting; instead, the fluids are dispensed by atmospheric pressure. With this system, the bag collapses as the fluids infiltrate and the chance of contamination is reduced because no air enters through the closed system. But, the collapse of the bag on infusion makes the determination of the amount of fluid left in the container difficult to evaluate. The system is closed to air so the chance of contamination is reduced and the safety for the patient is increased. In these containers, the fluids are easily transported and the chance for breakage is reduced (Figure 4-1). The storage requirements are reduced because the containers are more flexible and weigh less, but the need to inspect the container for possible contamination from tears or leaks is increased. Because of the chance of leaks and puncturing the container, writing directly on the container should not occur. The basic components of administration sets include a spike or piercing pin that is sharply tipped to allow for insertion into the solution container (Figure 4-2). This may be a vented spike as needed for open fluid containers or nonvented sets as needed for closed containers, as described earlier. The spike must remain sterile, so it is manufactured with a removable cover to protect its sterility. The spike has a flange so the fingers do not contaminate the actual spike during the piercing. The spike is an extension of the drop orifice and drip chamber. The drop orifice is found at the top of the drip chamber and determines the size and shape of the drop. These drops are calibrated for the infusion rate in drops per milliliter. This is called the drop factor used for calculations of flow rate. Primary infusion sets are available in macrodrip form, which allows 8 to 20 drops/mL to enter the drip chamber, and microdrip, also called the pediatric chamber, which allows 50 to 60 drops/mL to enter. The microdrip or minidrip is used when only small amounts of fluid are to be infused (Figure 4-3). Clamps, injection ports, and back-check valves may be found on the tubing, depending on the manufacturer (Figure 4-4). Clamps are used to compress the walls of the tubing to adjust flow rates by changing the size of the lumen of the tubing. The clamp may be on a roller, screw, or slide device, with the slide clamp being the least reliable method of compression. Roller and screw clamps may be adjusted in small increments to regulate the amount of fluid being infused. Injection ports are an access into the tubing and are used to add piggyback fluids to the primary set. These ports are usually located along the tubing at various sites, and small-lumened needles or needleless systems should be used for the secondary infusion to ensure the port will reseal following the secondary infusion. The backcheck valve is used to allow the primary infusion to continue when the secondary or piggyback infusion has completed. It also prevents the secondary infusion from entering the primary solution. Finally filters may be manufactured in the tubing or may be added to the tubing as needed (Figure 4-5). These filters are used to remove foreign particles such as bacteria, air, particulates, and the like from the infusion prior to the fluid entering the body. Primary infusion sets (Figure 4-2, p. 49) are also referred to as standard sets. These are selected by the correct infusion flow rate and the intended use. The most often used primary infusion set is the nonvented or universal set that is used for plastic bags of fluids. The primary line may also contain Y-connectors that allow secondary lines to be attached to the primary set. The drop size should be correctly selected for the time of infusion and the condition of the patient. The length of the tubing for the primary set should also be chosen by the size, condition, and ambulatory needs of the patient. The tubing should be long enough to allow the patient some activity while providing for the proper placement and securing of the needed equipment. Therefore, most tubing for primary administration sets is between 60 and 110 inches, usually about 80 inches. Again, remember that the manufacturer’s packaging label shows the drop size and the tubing length, and it states whether the tubing is vented and other possible additions to the tubing. In the case where needles or catheters are provided with the tubing, the gauge and length of the needle or catheter will also be shown on the label. The secondary administration set usually has shorter tubing (usually 32 to 42 inches) than the primary set because it is designed to insert into the primary set through the Y-connector or secondary insertion site. A needleless system or a small-gauged needle may be used to insert the secondary lines. When adding secondary sets, the set should either be lower than the container of primary fluids or should have a back-check valve to prevent the back flow of secondary fluids into the primary line (Figure 4-6). The ports used for secondary administration sets should ideally be punctured using a needleless or needle-protective device to prevent needlestick injuries and to ensure the resealing of the ports following administration of the secondary fluids. Needleless systems consist of a blunt-tipped plastic insertion tool found on the secondary tubing and a port for injection, found on the primary administration set, that opens on activation and immediately reseals when no longer in use (Figure 4-7). The use of this system eliminates the need to use needles and the potential for needlestick injuries, except during the initial insertion of the infusion line.
Basic Equipment and Supplies for Intravenous Therapy
TYPES OF CONTAINERS
Plastic or Closed Containers
TYPES OF IV ADMINISTRATION SETS
Basic Components Found in Administration Sets
Primary Infusion Sets
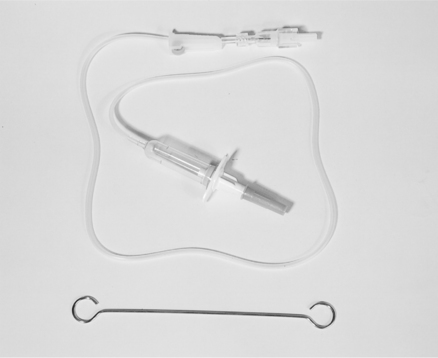
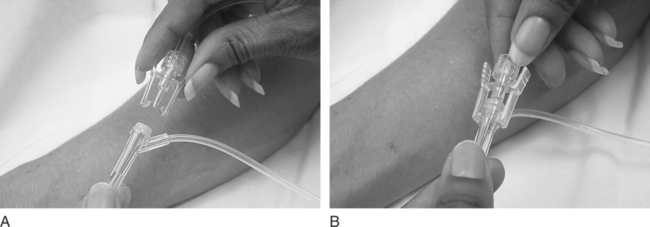
Secondary Administration Sets
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basic Equipment and Supplies for Intravenous Therapy