Chapter 14 Atrial Tachyarrhythmias in Congenital Heart Disease
Pathophysiology
Patients with congenital heart disease have a high prevalence of atrial tachycardias (ATs), particularly after they have undergone reparative or palliative surgical procedures. For macroreentrant ATs in adults with repaired congenital heart disease, three right atrial (RA) circuits are generally identified: lateral wall circuits with reentry around or related to the lateral atriotomy scar, septal circuits with reentry around an atrial septal patch, and typical atrial flutter (AFL) circuits using the cavotricuspid isthmus (CTI). Typical clockwise or counterclockwise isthmus-dependent AFL is the most common macroreentrant tachycardia in this patient population. Left atrial (LA) macroreentrant circuits are infrequent in this group of patients. Atrial macroreentry in the right free wall is the most common form of non–isthmus-dependent RA macroreentry (atypical flutter). Very complex or multiple reentry circuits can be seen after placement of an intraatrial baffle (Mustard or Senning correction for transposition of the great vessels) in an extremely dilated RA, after a Fontan procedure, and in patients with a univentricular heart.1,2
Anatomical factors promoting macroreentry in patients with congenital heart disease include abnormalities of the underlying cardiac anatomy, surgically created anastomoses, and atriotomy scars, resulting in anatomical barriers to impulse propagation. Additionally, extensive cardiac surgery and hemodynamic overload result in myocardial hypertrophy and diffuse areas of atrial scarring with surviving myocardial fibers embedded within scar areas, which provide the substrate for potential reentry circuits.3,4
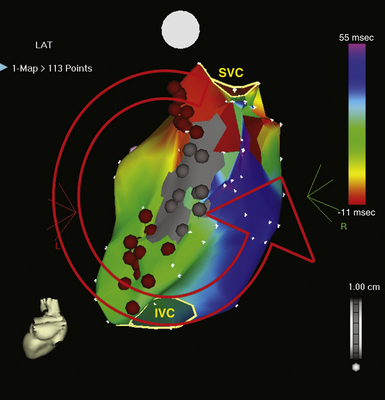
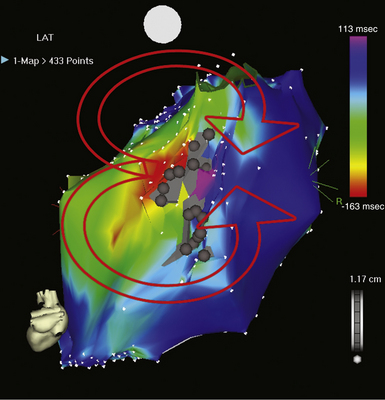
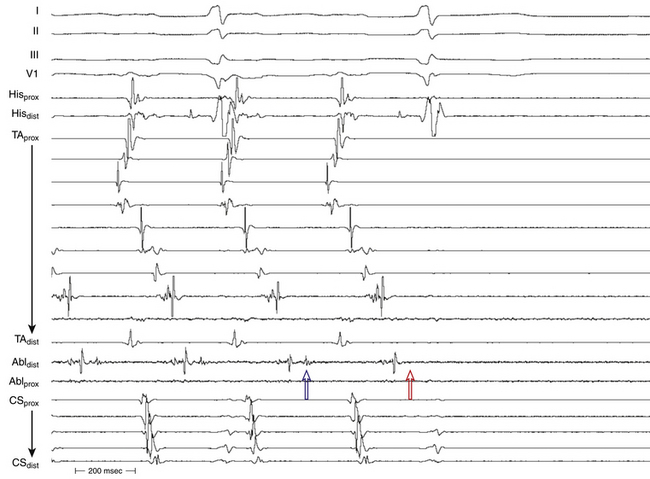
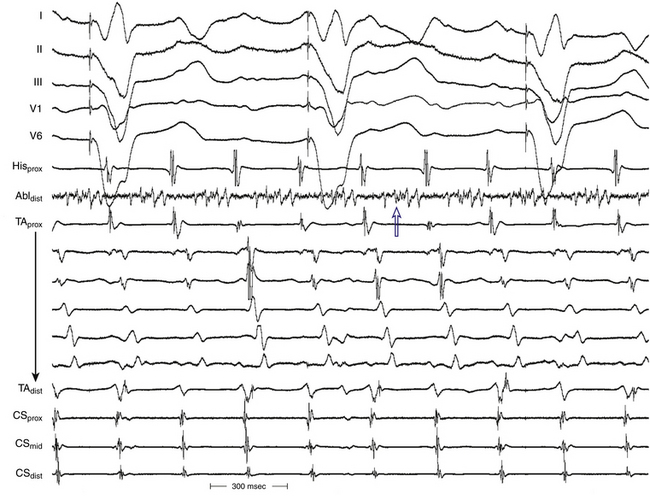
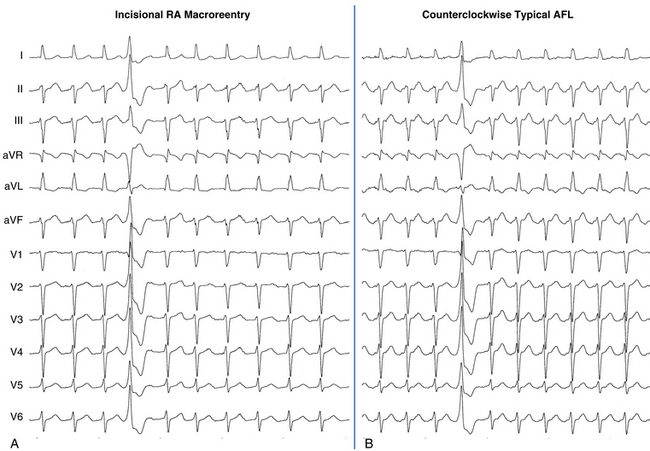
The best characterization of macroreentrant AT caused by atriotomy is activation around an incision scar in the lateral RA wall, with a main superoinferior axis (Fig. 14-1). This is a common problem in patients who have undergone surgery for congenital or valvular heart disease. The length, location, and orientation of the atriotomy incisions, as well as potential electrical conduction gaps across the atriotomy, are important determinants of arrhythmogenicity.5 Typically, the reentry circuit is located in the lateral RA wall. Not only does the central obstacle include the scar, but also functional block can magnify this obstacle to include the superior vena cava (SVC). The anterior RA wall is commonly activated superoinferiorly (descending activation pattern), as in counterclockwise typical AFL. However, the septal wall frequently lacks a clear-cut inferosuperior (ascending) activation pattern. Participation of the anterior RA wall in the circuit can be confirmed with entrainment mapping. A line of double potentials can be recorded in the lateral RA, extending superoinferiorly. Double potential separation can be more marked and demonstrate a voltage lower than in typical AFL. Narrow passages (isthmuses) in the circuit can be found between the SVC and the superior end of the atriotomy scar, between the inferior vena cava (IVC) and the inferior end of the atriotomy, between the atriotomy scar and the tricuspid annulus, between the atriotomy and the crista terminalis, or even within the scar itself (Fig. 14-2). These isthmuses can be areas of slow conduction. Stable pacing of the critical isthmus can be difficult or impossible in RA atriotomy tachycardia because of tachycardia interruption. Isthmus participation in the circuit is often proven by tachycardia interruption with catheter pressure (Fig. 14-3), as well as by tachycardia interruption and noninducibility after radiofrequency (RF) application in the area. A single, wide, fractionated electrogram can be recorded from the lower pivot point of the circuit (Fig. 14-4) in the low lateral RA, close to the IVC, and perhaps also from other isthmuses of the circuit. The line of double potentials or fractionated, low-voltage electrograms can also often be recorded in normal sinus rhythm, to allow tentative localization of the scar and the associated anatomical isthmuses.2
Typical AFL is also often associated with RA atriotomy. In fact, the single most common form of AT among patients with congenital heart disease appears to be isthmus-dependent AFL, particularly in patients with simpler anatomical lesions (e.g., tetralogy of Fallot, atrial and ventricular septal defects) (Fig. 14-5). Moreover, the CTI was found to be part of the reentrant circuit in approximately 70% of patients with postoperative intraatrial reentrant tachycardia. In one report, ablation of this isthmus alone resulted in elimination of the tachycardia in 27% of these patients. Factors such as an atriotomy or atrial fibrosis and hypertrophy serve as promoters for early development of the typical form of AFL.
Reentry circuits can also occur in the sinus node region, possibly as a result of injury related to the superior atrial cannulation site for the bypass pump. These circuits can be quite small, often manifesting as focal tachycardia in the sinus node region, and they frequently can be ablated in a single location without establishing a particular line of block.4
Focal mechanisms underlying postoperative AT have been rarely reported in this patient population. Nonautomatic focal ATs are predominantly found in adults, with most foci in the RA. The reasons behind this laterality are unknown. The mechanism underlying focal AT is unknown. Both triggered and microreentrant mechanisms have been suggested.6 Viable myocardial fibers embedded within areas of scar tissue, which play a pivotal role in the initiation and perpetuation of macroreentrant tachycardias, can also be the site of origin of a focal AT and thus play an important role in the pathogenesis of these ATs.3
Arrhythmias are also frequently observed in the early postoperative period after corrective surgery in children, occurring in 14% to 48% in the first few days after surgery. The most common arrhythmia in this period is junctional tachycardia, occurring in 5% to 10% of the operated children and usually self-limiting. Other supraventricular arrhythmias are also seen in 4%. The occurrence of early postoperative arrhythmias seems to be related to procedural factors of cardiac surgery, which are, in turn, related to the complexity of the congenital malformation. Early postoperative arrhythmias influence the long-term outcome of patients with congenital heart disease and have been found to be a predictor of late complications, such as ventricular dysfunction, late arrhythmias, and late mortality. Whether preventing these arrhythmias will influence the long-term survival of patients with congenital heart disease is unknown.7
Clinical Considerations
Epidemiology
Interestingly, compared with patients with structurally normal hearts, the incidence of atrial fibrillation (AF) in patients with congenital heart disease is relatively low. AF is less common than one would anticipate, especially considering the often extremely dilated RA in this patient population.8 AF is typically associated predominantly with markers of left-sided heart disease (i.e., lower left ventricular ejection fraction and LA dilation) and is most commonly seen in patients with congenital aortic stenosis, mitral valve disease, palliated single ventricles, or end-stage heart disease.1,9
Atrial Septal Defect
Macroreentrant ATs are the most frequent arrhythmias encountered in patients with secundum and sinus venosus atrial septal defects. In the absence of surgical repair, the prevalence of supraventricular arrhythmias increases with age. These arrhythmias have been reported in 20% of these patients at the age of 40 years, and typical AFL is the most common circuit. In the presence of atriotomy incisions, sutures, or patches, non–isthmus-dependent macroreentrant circuits can occur or coexist with typical AFL. Common substrates include macroreentry along the lateral RA wall and double-loop or figure-of-8 circuits. The septal patch itself is rarely a critical conduction obstacle. Surgical closure of an atrial septal defect during childhood provides a substantially lower incidence of arrhythmias. In contrast, surgical closure at adult age is far less effective; approximately 60% of these patients continue to have atrial arrhythmias during follow-up after surgery. The impact of transcatheter atrial septal defect closure on atrial arrhythmias is less clear. In one series, all patients with persistent arrhythmias remained in AF or AFL after closure.7,8,10
Univentricular Hearts with Fontan Palliation
Among patients with congenital heart disease, the incidence of macroreentrant AT is highest (16% to 56%) among older patients who have undergone older-style Fontan (atriopulmonary anastomosis) operations, in which extensive suture lines and long-term hemodynamic stress result in marked atrial hypertrophy and fibrosis.4 The incidence of atrial tachyarrhythmias appears lower in patients with total cavopulmonary connections in comparison with classical atriopulmonary connections. Overall, the most common arrhythmia is atrial macroreentry. Tachycardia circuits may be complex or multiple. Single circuits that are quite amenable to catheter ablation can occasionally be encountered.11
Tetralogy of Fallot
ATs occur commonly (12% to 34%) during extended follow-up after tetralogy of Fallot repair. The observed prevalence of atrial arrhythmias (20.1%) is modestly higher than that of ventricular arrhythmias (14.6%).12 The most common atrial circuit is typical clockwise or counterclockwise AFL. Other circuits often involve the lateral RA wall and may be multiple, often with a double-loop type of reentry. Nonautomatic focal ATs most commonly arise adjacent to suture points, with radial spread of activation.
The prevalence of AF increases with advancing age. In the first few decades of life, AF is far less common than macroreentrant AT, but it becomes more common (more than 30%) than macroreentrant AT after 55 years of age.12
d-Transposition of the Great Arteries
The Mustard or Senning atrial switch procedures were performed from the early 1960s until approximately 1985 as the major long-term surgical palliation procedures for young children having d-transposition of the great arteries. Hence, there is a population of patients in their late 20s to early 50s who have undergone these operations and who are at great risk of having supraventricular arrhythmias (15% to 48%), with similar rates in patients with Mustard and Senning baffles.4,11 Most ATs in this patient group are typical AFL, but non–isthmus-dependent macroreentrant ATs with critical zones of slow conduction between a suture line and the SVC orifice, mitral valve annulus, and pulmonary vein orifice have all been described. Focal ATs adjacent to suture lines are also not uncommon. Currently, arterial switch surgery has supplanted atrial redirection as the procedure of choice for d-transposition of the great arteries, and it has been associated with a lower risk of arrhythmias.8,10
Reports suggest that atrial tachyarrhythmias are important contributors to sudden death. Contributing factors may include longer cycle lengths (CLs) than in typical AFL (favoring 1:1 conduction), impaired atrioventricular (AV) transport with failure to augment right ventricular filling rates during tachycardia, systemic ventricular dysfunction, and subendocardial ischemia resulting from right coronary circulation irrigation of a systemic ventricle.8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree