Chapter 148 Atherosclerosis
 Diagnostic Summary
Diagnostic Summary
• Characteristically associated with high blood pressure, weak pulse, and wide pulse pressure
• Symptoms and signs depend on the arteries involved and degree of obstruction: angina, leg cramps (intermittent claudication), gradual mental deterioration, weakness or dizziness
• May also be without symptoms
 General Considerations
General Considerations
Understanding Atherosclerosis
Structure of an Artery
An artery is divided into three major layers:
• The intima, representing the endothelium or internal lining of the artery. It consists of a layer of endothelial cells. Glycosaminoglycans (GAGs) line the exposed endothelial cells to protect them from damage as well as to promote repair. Beneath the surface cells is the internal elastic membrane, composed of a layer of GAGs and other ground substance compounds, which supports the endothelial cells and separates the intima from the smooth muscle layer.
• The media consists primarily of smooth muscle cells. Interposed among the cells are GAGs and other ground substance structures that provide support and elasticity to the artery.
• The adventitia, or external elastic membrane, consists primarily of connective tissue including GAGs; it provides structural support and elasticity to the artery.
The Process of Atherosclerosis
The lesions of atherosclerosis are initiated in response to injury to or a disruption of the normal functioning of the arterial endothelium.1 The progression of atherosclerosis is detailed as follows:
1. The initial step is damage or dysfunction of the vascular endothelium. Damage results from weakening of the GAG layer, which protects the endothelial cells, due to the same factors that ultimately damage the endothelial cells (insulin resistance, reactive oxygen and nitrogen species, impaired repair processes, heavy metal toxicity, hyperhomocysteinemia, and inhibition of either nitric oxide production or availability).
2. Once the endothelial cells have been sufficiently damaged, sites of injury become more permeable to plasma constituents, especially lipoproteins. The binding of lipoproteins to GAGs leads to a breakdown in the integrity of the ground substance matrix and causes an increased affinity for cholesterol. Simultaneously, monocytes, T lymphocytes, and platelets adhere to the damaged area, where they release growth factors that stimulate smooth muscle cells to migrate from the media into the intima and replicate.
3. Local concentration of lipoproteins, monocytes, and platelets leads to the migration of smooth muscle cells from the media into the intima, where they undergo proliferation. Smooth muscle cells dump cellular debris into the intima, leading to the further development of plaque.
4. Formation of a fibrous cap (consisting of collagen, elastin, and GAGs) over the intimal surface occurs. Fat and cholesterol deposits accumulate.
5. Plaque continues to grow until eventually it either blocks the artery directly or ruptures to form a clot that travels the general circulation until it occludes a blood vessel. Plaque instability is associated with a significantly greater risk for myocardial infarction (MI) or stroke.1 Thus, targeting plaque stabilization appears to be more clinically important than simply enlarging the lumen.
Causative Factors
Prevention of CVD involves reducing and, when possible, eliminating various risk factors. Risk factors are divided into two primary categories: major risk factors and other risk factors. Box 148-1 lists the major risk factors. Risk for a heart attack increases with the number of major risk factors, as Table 148-1 shows.
TABLE 148-1 Association of Risk Factors with the Incidence of Atherosclerosis
| MAJOR RISK FACTORS | INCREASE IN INCIDENCE |
|---|---|
| Presence of one of the major risk factors | 30% |
| High cholesterol and high blood pressure | 300% |
| High cholesterol and a smoker | 350% |
| High blood pressure and a smoker | 350% |
| Smoker, high blood cholesterol, and high blood pressure | 720% |
In addition to these well-accepted major risk factors, numerous others have occasionally been shown to be more significant. Box 148-2 lists these additional risk factors. It is also important to develop a strategic approach to plaque stabilization by addressing endothelial dysfunction as well as increased local and systemic inflammation, increased reactive oxygen species, the activation of mast cells, and the infiltration and activation of macrophages.
BOX 148-2 Other Risk Factors for Atherosclerosis
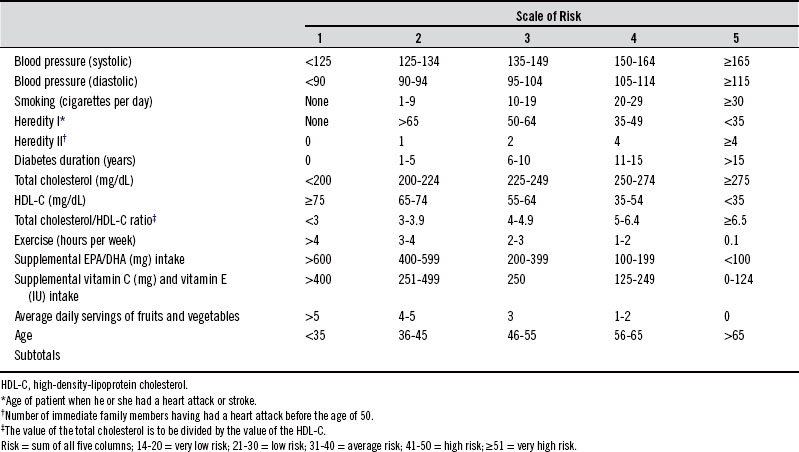
Determining a Patient’s Risk
To help determine a patient’s overall risk for having a heart attack or stroke, the following risk-determinant scale may prove useful. Although this risk assessment does not take into consideration several other important factors—such as the level of C-reactive protein, lipoprotein(a) or Lp(a), fibrinogen, and coping style—the score provides a good indication of a patient’s risk for a heart attack or stroke (Table 148-2).
Clinical Evaluation
The complete cardiovascular assessment can include the tests listed in Box 148-3.
Risk Factors
Smoking
Cigarette smoking is perhaps the most important risk factor for CVD, because statistical evidence reveals smokers have a 70% greater risk of death from CVD than nonsmokers.2 The more cigarettes smoked and the longer the period of years a person has smoked, the greater the risk of dying from a heart attack or stroke. Overall, the average smoker dies 7 to 8 years sooner than the nonsmoker.
Tobacco smoke contains more than 4000 chemicals, of which more than 50 substances have been identified as carcinogens. These chemicals are extremely damaging to the cardiovascular system. Specifically, these chemicals are carried in the bloodstream on low-density lipoprotein cholesterol (LDL-C), where they either damage the lining of the arteries directly or they damage the LDL-C molecule, which then damages the arteries. An elevated LDL-C level worsens the effect of smoking on the cardiovascular system because more cigarette toxins travel through it. Smoking contributes to elevated cholesterol presumably by damaging feedback mechanisms in the liver, which control how much cholesterol is being manufactured.3 Smoking also promotes platelet aggregation and elevated fibrinogen levels, two other important independent risk factors for CVD. In addition, it is a well-documented fact that cigarette smoking is a contributing factor to high blood pressure.4
Even passive exposure to cigarette smoke is damaging to cardiovascular health, because convincing evidence links environmental (secondhand or passive) smoke to heart disease mortality and morbidity. Analysis of ten epidemiologic studies indicates a consistent dose-response effect related to exposure.5 Evidence indicates that nonsmokers appear to be more sensitive to smoke, including its deleterious effects on the cardiovascular system. Environmental tobacco smoke actually has a higher concentration of some toxic constituents. Pathophysiologic and biochemical data after short- and long-term environmental tobacco smoke exposure show changes in the lining of the arteries and in platelet function as well as exercise capacity, similar to those in active smoking. In summary, passive smoking is a relevant risk factor for CVD. In the United States it is estimated that more than 37,000 coronary heart disease (CHD) deaths each year are attributable to environmental smoke.
The magnitude of risk reduction achieved by smoking cessation in patients with CVD is quite significant. Results from a detailed meta-analysis showed a 36% reduction in relative risk of mortality for patients with CAD who quit compared with those who continued smoking.6
Various measures—including nicotine-containing skin patches or chewing gum, acupuncture, and hypnosis—have all been shown to provide some benefit in helping patients to quit smoking, but not much. In a systematic review of the efficacy of interventions intended to help people to stop smoking, data were analyzed from 188 randomized controlled trials.7 Encouragement to stop smoking by physicians during a routine office call resulted in a 2% cessation rate after 1 year. Supplementary measures such as follow-up letters or visits had an additional effect. Behavioral modification techniques such as relaxation, rewards and punishments, and avoiding trigger situations in group or individual sessions led by a psychologist had no greater effect than the 2% rate achieved by simple advice from a physician. Eight studies with acupuncture have produced an overall effectiveness rate of roughly 3%. Hypnosis has been judged to be ineffective even though trials have shown a success rate of 23%. Hypnosis was judged to be ineffective because no biochemical marker was used to accurately determine effectiveness. Nicotine replacement therapy (gum or patch) is effective in about 13% of smokers who seek help in quitting. All together, these results are not encouraging. It appears that the best results occur when people quit “cold turkey.” Box 148-4 lists ten tips to help patients stop smoking.
BOX 148-4 Tips to Help Patients Stop Smoking
• List all the reasons why you want to quit smoking and review them daily.
• Set a specific day to quit, tell at least ten friends that you are going to quit smoking, and then . . . DO IT!
• Throw away all cigarettes, butts, matches, and ashtrays.
• Use substitutes. Instead of smoking, chew on raw vegetables, fruits, or gum. If your fingers seem empty, play with a pencil.
• Realize that 40 million Americans have quit. If they can do it, so can you!
• Visualize yourself as a nonsmoker with a fatter pocketbook, pleasant breath, unstained teeth, and the satisfaction that comes from being in control of your life.
• Join a support group. Call the local branch of the American Cancer Society and ask for referrals. You are not alone.
• When you need to relax, perform deep breathing exercises rather than reaching for a cigarette.
• Avoid situations that you associate with smoking.
• Each day, reward yourself in a positive way. Buy yourself something with the money you have saved or plan a special reward as a celebration for quitting.
Elevated Blood Cholesterol Levels
Cholesterol is transported in the blood by lipoproteins. The major categories of lipoproteins are very-low-density lipoprotein (VLDL), LDL, and HDL. Because VLDL and LDL are responsible for transporting fats (primarily triglycerides and cholesterol) from the liver to body cells, whereas HDL is responsible for returning fats to the liver, elevations of either VLDL or LDL are associated with an increased risk for developing atherosclerosis, the primary cause of a heart attack or stroke. In contrast, elevations of HDL-C are associated with a low risk of heart attacks.
The ratios of total cholesterol to HDL-C and LDL-C to HDL-C are referred to as the cardiac risk factor ratios because they reflect whether cholesterol is being deposited into tissues or broken down and excreted. The ratio of total cholesterol to HDL-C should be no higher than 4.2, and the ratio of LDL-C to HDL-C should be no higher than 2.5. The risk of heart disease can be reduced dramatically by lowering LDL-C while simultaneously raising HDL-C levels: For every 1% drop in the LDL-C level, the risk of a heart attack drops by 2%. Conversely, for every 1% increase in HDL-C level, the risk for a heart attack drops 3% to 4%.8
Further Refinement of Determining Risk. Although LDL-C is referred to as “bad cholesterol,” there are some forms that are worse than others. For example, oxidized LDL-C is a persistent proinflammatory trigger for the progression of atherosclerosis and plaque rupture. LDL-C molecules of higher density are associated with greater risk than larger, less dense molecules. Small, dense LDL-C has a greater content of apolipoprotein CIII (apo CIII); in addition, apoB particles are more atherogenic than larger, less dense LDL-Cs and are markers for CVD risk.9 In a small trial of nondiabetic subjects, researchers determined that these smaller particles were more heavily and preferentially glycated over the larger, more buoyant LDL-C particles, strongly suggesting an explanation for why these particles are more likely to participate in atherogenesis and highlighting the importance of avoiding hyperglycemia and excessive glycation.10
Another marker that deserves mention is Lp(a), a plasma lipoprotein whose structure and composition closely resemble those of LDL-C but with an additional molecule of an adhesive protein called apolipoprotein(a). Elevated plasma levels of Lp(a) are an independent risk factor for CHD, particularly in those patients with elevated LDL-C levels. In fact, in one analysis a high level of Lp(a) was shown to carry with it a ten times greater risk for heart disease than an elevated LDL-C level.11 That is because LDL-C on its own lacks the adhesive apolipoprotein(a). As a result, LDL-C does not easily stick to the walls of the artery. Actually, a high LDL-C level carries less risk than a normal or even low LDL-C with a high Lp(a). Levels of Lp(a) below 20 mg/dL are associated with a low risk of heart disease; levels between 20 and 40 mg/dL are associated with a moderate risk; and levels above 40 mg/dL are associated with an extremely high risk of heart disease.
Elevations of Triglycerides. In the past, the relation between hypertriglyceridemia and CHD has been uncertain. However, a large body of accumulating evidence indicates that hypertriglyceridemia (HTG) is an independent risk factor for CVD. Multivariate analysis of 8-year follow-up data from the large-scale Prospective Cardiovascular Munster Study found HTG to be an independent risk factor for major coronary events after controlling for LDL-C and HDL-C.12 HTG combined with elevated LDL-C and a high LDL-C:HDL-C (>5) increased the risk for a CHD event by approximately sixfold. Similarly, a large meta-analysis of 17 prospective trials reported HTG to be an independent risk factor for CVD.13 In this study, an 88 mg/dL (1.0 mmol/L) increase in plasma triglyceride levels significantly increased the relative risk of CVD by approximately 30% in men and 75% in women; the corresponding rates were somewhat lower (14% and 37%, respectively) but still statistically significant after adjustment for HDL-C level.
FCH and FHTG result in similar defects to FH. In FCH, the basic defect appears to be an accelerated production of VLDL in the liver. These individuals may have only a high blood triglyceride level, only a high cholesterol level, or both. In FHTG there is only an elevation in blood triglyceride levels, and HDL-C levels tend to be low. The defect in FHTG is that the VLDL particles made by the liver are larger than normal and carry more triglycerides. FHTG is made worse by diabetes, gout, and obesity.
Diabetes
Atherosclerosis is one of the key underlying factors in the development of many chronic complications of diabetes. Individuals with diabetes have a twofold to threefold higher risk of dying prematurely of heart disease or stroke than those who are not diabetic, and 55% of deaths in diabetic patients are caused by CVD. However, even mild insulin resistance and poor glucose control have both been shown to have a dramatic impact on the incidence and progression of CVD. For more information, see Chapter 161.
High Blood Pressure
Elevated blood pressure is often a sign of considerable atherosclerosis and a major risk factor for heart attack or stroke. In fact, the presence of hypertension is generally regarded as the most significant risk factor for stroke. For more information, see Chapter 174.
Physical Inactivity
A sedentary lifestyle is another major risk factor for CVD. Physical activity refers to “bodily movement produced by skeletal muscles that requires energy expenditure” and produces healthy benefits. Exercise, a type of physical activity, is defined as “a planned, structured, and repetitive bodily movement done to improve or maintain one or more components of physical fitness.” Physical inactivity denotes a level of activity less than that needed to maintain good health. Physical inactivity characterizes most Americans, because roughly 54% of adults report little or no regular leisure physical activity, and there is also a sharp decline in regular exercise among children and adolescents.1
Other Risk Factors
In addition to the major risk factors for CVD (i.e., smoking, elevations in cholesterol, elevated blood pressure, diabetes, and physical inactivity/obesity), a number of other factors have, on occasion, been shown to be more significant than the so-called major risk factors. In fact, more than 300 different risk factors have been identified, including those listed in Box 148-2.
Although there is considerable evidence that all of these risk factors and more can play significant roles in the pathogenesis of atherosclerosis, much of the current research has focused on the central roles of inflammatory processes and insulin resistance.14 Inflammatory mediators influence many stages of atheroma development, from initial leukocyte recruitment to eventual rupture of the unstable atherosclerotic plaque. In particular, C-reactive protein (CRP), an acute-phase reactant that reflects different degrees of inflammation, has been identified as an independent risk factor for CAD. Although the CRP level has shown to be a stronger predictor of cardiovascular events than the LDL-C level, screening for both biological markers provides better prognostic information than screening for either alone.15
Elevations in CRP are closely linked to insulin resistance and the metabolic syndrome.16 The diagnostic criteria for the metabolic syndrome comprise the presence of at least three of the following metabolic risk factors in one person:
• Central obesity (a waist-to-hip ratio >1 for men; >0.8 for women)
• Atherogenic dyslipidemia (mainly triglycerides >150 mg/dL and low HDL-C [<40 mg/dL in men and <50 mg/dL in women])
• Hypertension (≥130/85 mm Hg)
• Insulin resistance or glucose intolerance (fasting blood sugar levels >101 mg/dL)
• Prothrombotic state (e.g., high fibrinogen or plasminogen activator inhibitor in the blood)
• Proinflammatory state (e.g., elevated high-sensitivity CRP in the blood)
 Therapeutic Considerations—General Guidelines
Therapeutic Considerations—General Guidelines
Prevention of a heart attack or stroke involves reducing risk factors. The major risk factor of smoking was discussed earlier, while obesity, physical inactivity, diabetes, and hypertension are detailed in separate chapters. There is significant evidence that simply adopting a healthy diet and lifestyle dramatically reduces CVD-related mortality. In a prospective trial enrolling over 20,000 men and women, it was found that the combination of four healthy behaviors (nonsmoking, not physically inactive, moderate alcohol intake, and a plasma vitamin C indicative of at least 5 servings of fruit and vegetables per day) reduced total mortality fourfold compared with those who had none of these behaviors.17
Diet—General Guidelines
The dietary program detailed in Chapter 44 features a comprehensive dietary approach for both the prevention and treatment of CVD as well as the improvement of blood lipid profiles. In particular, it is important to reduce the intake of saturated fat and trans fatty acids while increasing the consumption of vegetables, fruit, dietary fiber, monounsaturated fats, and omega-3 fatty acids. An important dietary goal is to improve the structure and composition of cell membranes by making available essential structural components like the monounsaturated and omega-3 fatty acids and by preventing oxidative and free radical damage to these structures by consuming a high level of antioxidants and phytochemicals.
One of the most widely studied dietary interventions in CAD is the traditional “Mediterranean diet,”18 which reflects food patterns typical of some Mediterranean regions in the early 1960s, such as Crete, parts of the rest of Greece, and southern Italy.
Unfortunately, the modern Mediterranean diet has deviated significantly from its healthful origin.
The original Mediterranean diet had the following characteristics:
• Olive oil is the principal source of fat.
• The diet centers on an abundance of plant food (fruits; potatoes, beans, and other vegetables; breads; pasta; nuts; and seeds).
• Foods are minimally processed, and people focus on seasonally fresh and locally grown foods.
• Fresh fruit is the typical daily dessert, with sweets containing concentrated sugars or honey consumed only a few times a week at the most.
• Dairy products (principally cheese and yogurt) are consumed daily in low to moderate amounts.
• Poultry and eggs are consumed in moderate amounts (up to four times a week) or not at all.
• Red meat is consumed in low amounts.
• Wine is consumed in low to moderate amounts, normally with meals.
In one study, the effect of the Mediterranean diet on endothelial function and vascular inflammatory markers was studied in patients with the metabolic syndrome.19 Patients in the intervention group were instructed to follow the Mediterranean diet and received detailed advice on how to increase their daily consumption of whole grains, fruits, vegetables, nuts, and olive oil; patients in the control group followed the American Heart Association (AHA) diet. After 2 years, patients following the Mediterranean diet consumed more foods rich in monounsaturated fat, polyunsaturated fat, and fiber and had a lower ratio of omega-6 to omega-3 fatty acids. Compared with patients consuming the control diet, patients consuming the intervention diet had significantly reduced serum concentrations of CRP and other inflammatory mediators; improved endothelial function; and greater weight loss.
Although several components of the Mediterranean diet deserve special mention, it is important to stress that the total benefits reflect an interplay among many beneficial compounds rather than any single factor.20
In addition, it is critical to follow a low glycemic diet. A prospective trial with more than 48,000 participants highlighted the importance of good glycemic control for preventing CVD. Following a study population for an average of 8 years, researchers found that the consumption of foods with a high glycemic load increased the risk of CHD in women by 68%; women in the highest glycemic-load quartile had a relative risk of 2.2 for CHD compared with those in the lowest quartile.21
Olive Oil and Omega-3 Fatty Acids
One of the most important aspects of the Mediterranean diet may be the combination of olive oil (a source of monounsaturated fats and antioxidants) and the intake of omega-3 fatty acids. Olive oil consists not only of monounsaturated fatty acid (oleic acid) but also of several antioxidant agents that may also account for some of its health benefits. In addition to a mild effect in lowering LDL-C and triglycerides, olive oil increases the HDL-C level and helps prevent LDL-C from being damaged by free radicals.22
In addition to olive oil, the benefits of the longer-chain omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahaexanoic acid (DHA) for cardiovascular health has been demonstrated in more than 300 clinical trials. These fatty acids exert considerable benefits in reducing the risk for CVD. Supplementation with EPA and DHA has little effect on cholesterol levels but does lower triglyceride levels significantly, as well as producing a myriad of additional beneficial effects including reduced platelet aggregation; improved endothelial function and arterial flexibility; improved blood and oxygen supply to the heart; and a mild effect in lowering blood pressure by vasodilation, and promotion of sodium excretion.23
The levels of EPA and DHA within red blood cells have been shown to be highly significant predictors of heart disease. This laboratory value has been termed the omega-3 index. An omega-3 index of 8% was associated with the greatest protection, whereas an index of 4% was associated with the least. In one analysis, the omega-3 index was shown to be the most significant predictor of CAD compared with CRP; total cholesterol, LDL-C, or HDL-C; and homocysteine. Researchers subsequently determined that a total of a combined 1000 mg of EPA and DHA daily is required to achieve or surpass the 8% target of the omega-3 index.24,25
The findings with the omega-3 index are not surprising, because a wealth of information has documented a clear relationship between dietary intake omega-3 fatty acids and the likelihood of developing CHD: the higher the omega-3 fatty acid intake, the lower the likelihood of CHD. It has been estimated that raising the levels of long-chain omega-3 fatty acids through diet or supplementation may reduce overall cardiovascular mortality by as much as 45%.26,27
In general, for preventive effects against CVD, the dosage recommendation is 1000 mg of EPA plus DHA a day; for lowering triglycerides, the dosage is 3000 mg. In a double-blind study, after 8 weeks of supplementation, a daily dosage of 3.4 g EPA plus DHA lowered triglycerides by 27%, while a lower dosage of 0.85 g had no effect. These results clearly indicate that the effective dosage for lowering triglycerides with fish oils requires dosages of 3 g EPA plus DHA a day.28
Although the longer-chain omega-3 fatty acids exert more pronounced effects than alpha-linolenic acid, the shorter omega-3 fatty acid from vegetable sources, it is important to point out that the two populations with the lowest rates of heart attack have a relatively high intake of alpha-linolenic acid: the Japanese who inhabit Kohama Island and the inhabitants of Crete.29,30 Typically, Cretans have a threefold higher serum concentration of alpha-linolenic acid than members of other European countries, owing to their frequent consumption of walnuts and the vegetable purslane.29 Of course, another important dietary factor in both the Kohamans and Cretans is their use of oleic acid–containing oils. However, although the oleic acid content of the diet offers some degree of protection, the rates of heart attack among the Kohamans and Cretans are much lower than those in populations that consume only oleic acid sources and little alpha-linolenic acid. The intake of alpha-linolenic acid is viewed as a more significant protective factor than oleic acid.
Nuts and Seeds
Higher consumption of nuts and seeds has been shown to significantly reduce the risk of CVD in large epidemiologic studies including the Nurses’ Health Study, the Iowa Health Study, and the Physicians’ Health Study.31 Researchers estimate that substituting nuts for an equivalent amount of carbohydrates in an average diet resulted in a 30% reduction in heart disease risk. Researchers calculated an even more impressive risk reduction, 45%, when fat from nuts was substituted for saturated fats (found primarily in meat and dairy products). Nuts have a cholesterol-lowering effect, which partly explains this benefit, but they are also a rich source of arginine. By increasing nitric oxide levels, arginine may help to improve blood flow, reduce blood clot formation, and improve blood fluidity (the blood becomes less viscous and therefore flows through blood vessels more easily).
Walnuts appear to be especially beneficial because they are also a rich source of both antioxidants and alpha-linolenic acid. In one study, hypercholesterolemic men and women were randomized to a cholesterol-lowering Mediterranean diet and a diet of similar energy and fat content in which walnuts replaced approximately 32% of the energy from monounsaturated fat (olive oil). Participants followed each diet for 4 weeks. Compared with the Mediterranean diet, the walnut diet improved endothelial cell function (it increased endothelium-dependent vasodilation and reduced levels of vascular cell adhesion molecule-1). The walnut diet also significantly reduced total cholesterol (–4.4%) and LDL-C (–6.4%).32
Vegetables, Fruits, and Red Wine
An important contributor to the benefits noted with the Mediterranean diet is the focus on carotene- and flavonoid-rich fruits, vegetables, and beverages (e.g., red wine). Numerous population studies have repeatedly demonstrated that a higher intake of dietary antioxidants significantly reduces the risk of heart disease and stroke. Higher blood levels of antioxidant nutrients are also associated with lower levels of CRP.33 The importance of antioxidant intake in the prevention and treatment of CAD is discussed further later.
Two valuable sources of antioxidants in the Mediterranean diet are tomato products and red wine. Tomatoes are a rich source of the carotene lycopene. In large clinical studies evaluating the relationship between carotene status and heart attack (acute MI), lycopene but not beta-carotene was shown to be protective. Lycopene exerts greater antioxidant activity compared with beta-carotene in general but specifically against LDL-C oxidation.34
The cardiovascular protection offered by red wine is popularly referred to as the “French paradox.” Because the French consume more saturated fat than people in the United States and the United Kingdom yet have a lower incidence of heart disease, red wine consumption has been suggested to be the reason. Presumably this protection is the result of flavonoids and other polyphenols in red wine that protect against oxidative damage to LDL-C as well as helping to reduce the levels of inflammatory mediators.19,35 However, moderate alcohol consumption alone has also been shown to be protective in some studies by exerting positive effects on ratios of HDL-C to LDL-C and CRP as well as levels of fibrinogen, although red wine typically exerts the most significant effects.36
The major benefits of red wine consumption in protecting against CVD may ultimately be the effects that the polyphenols have on improving endothelial cell function. In one study, 30 male patients with CHD were randomly assigned to take a red grape polyphenol extract (600 mg) or placebo. Flow-mediated dilation was measured after fasting and 30, 60, and 120 minutes after intake of the grape extract or placebo. The intake of the red grape polyphenol extract caused an increase in flow-mediated dilation, peaking at 60 minutes, which was significantly higher than the baseline values and the corresponding values at 60 minutes after the intake of placebo (4.52% vs 2.64%).37
The consumption of green tea, like that of red wine, has also been shown in population studies to be associated with a reduced risk for CVD. As with red wine, much of the benefit of green tea consumption may be the result of several different mechanisms, including improving endothelial cell function. Green tea polyphenols (catechins) have been shown to decrease the oxidation of LDL-C, lower LDL-C levels, and improve the LDL-C:HDL-C ratio. In vitro studies have shown a dose-dependent response with green tea polyphenols in reducing several biomarkers associated with atherosclerosis and ischemia, including the inhibition of the endothelial cell–derived vascular cell adhesion molecule-1 as well as angiotensin II, platelet derived growth factor-BB, apolipoprotein E, and inducible nitric oxide synthase.38
Another mechanism of action for red wine and green tea polyphenols involves inhibiting the formation of new blood vessels within the vascular lesion. During the angiogenic process, new blood vessels develop from the existing microvascular bed. The initial event involves dilation of an existing blood vessel followed by an increase in vascular permeability and the degradation of extracellular matrices. This process allows endothelial cells to migrate into the lesion and proliferate, and these events are followed by the maturation of new blood vessels. The angiogenic process is controlled by two major proangiogenic factors: matrix metalloproteinases (MMPs), which degrade extracellular matrices, and vascular endothelial growth factor (VEGF), which strongly stimulates endothelial cell migration and proliferation and the formation of new blood vessels. Both red wine and green tea polyphenols have been shown to inhibit this process in vitro at concentrations conceivably achievable with oral intake.38
Foods and beverages rich in antioxidant content have shown benefit in fighting atherosclerosis. Pomegranate (Punica granatum) juice appears to be particularly useful. It is remarkably rich in antioxidants, such as soluble polyphenols, tannins, and anthocyanins. Animal research has indicated that components of pomegranate juice can retard atherosclerosis, reduce plaque formation, and improve arterial health. Human clinical studies have supported the role of pomegranate juice in benefiting heart health.39–41
In a randomized placebo-controlled double-blind study, the daily consumption of pomegranate juice for 3 months was evaluated for its effect on myocardial perfusion in 45 patients who had CHD and myocardial ischemia. Patients were randomly assigned into one of two groups: a pomegranate juice group (240 mL/day) or a placebo group, which drank a beverage of similar caloric content, amount, flavor, and color. Participants underwent electrocardiographic-gated myocardial perfusion single-photon emission computed tomographic scintigraphy at rest and during stress at baseline and 3 months. After 3 months, the extent of stress-induced ischemia decreased in the pomegranate group but increased in the control group. This benefit was observed without changes in cardiac medications, blood sugar, hemoglobin A1c, weight, or blood pressure in either group.39
In another study, 10 patients with carotid artery stenosis (CAS) were evaluated to gauge the effect of pomegranate juice consumption on the progression of carotid lesions and changes in oxidative stress and blood pressure. The patients were supplemented with pomegranate juice (50 mL) for 1 year, and 5 continued for up to 3 years. In the control group (which did not consume pomegranate juice), common carotid intimal-medial thickness (IMT) increased by 9% during 1 year, whereas pomegranate juice consumption resulted in a significant IMT reduction, by up to 30%, after 1 year. Compared with values obtained before pomegranate juice consumption, the patients’ serum paraoxonase 1 (PON 1) activity was increased by 83%, whereas serum LDL-C basal oxidative state and LDL-C susceptibility to copper ion–induced oxidation were both significantly reduced, by 90% and 59%, respectively, after 12 months. Furthermore, serum levels of antibodies against oxidized LDL-C were decreased by 19%; in parallel, serum total antioxidant status was increased by 130% after 1 year of pomegranate juice consumption. Systolic blood pressure was reduced after 1 year of pomegranate juice consumption by 21%. For all studied parameters, the maximal effects were observed after 1 year of pomegranate juice consumption.40
 Therapeutic Considerations—Lowering Cholesterol
Therapeutic Considerations—Lowering Cholesterol
Although the data are clear that statin drugs can produce decreases in total mortality, cardiovascular events, hospitalizations, and the need for revascularization procedures in high-risk patients, the debate remains whether statin therapy represents the optimal treatment approach to the primary prevention of CAD in patients with only the risk factor of elevated LDL-C, especially in light of the growing acceptance of risk factors like CRP and the emerging role of nutrition.42,43 For example, one interesting study compared the “portfolio diet,” comprising plant-based cholesterol-lowering foods, to lovastatin.44 The participants were randomly assigned to undergo one of three interventions on an outpatient basis for 1 month: a diet low in saturated fat, based on milled whole-wheat cereals and low-fat dairy foods; the same diet plus lovastatin, 20 mg/day; or a diet high in plant sterols (1 g/1000 kcal), soy protein (21.4 g/1000 kcal), viscous fibers (9.8 g/1000 kcal), and almonds (14 g/1000 kcal). The control, statin, and dietary portfolio groups had mean (SE) decreases in LDL-C of 8%, 30.9%, and 28.6%, respectively. Respective reductions in CRP were 10%, 33.3%, and 28.2%. This study and subsequent studies showed that by diversifying the cholesterol-lowering components in the same dietary portfolio, the effectiveness of diet as a treatment of hypercholesterolemia was increased. Moreover, results comparable with those of a statin drug (with lipid-lowering effects similar to statins in terms of both LDL-C and size of LDL-C) were produced.45,46
Whereas individual dietary changes may produce benefit in improving blood lipids, the best clinical approach is to incorporate a broad-spectrum dietary approach that incorporates a wide array of dietary components shown to have a positive impact on lipid levels. For example, although a meta-analysis of 27 randomized controlled trials in which isolated soy protein supplementation was the only intervention demonstrated that soy protein supplementation was associated with a significant dose-dependent reduction in mean serum total cholesterol, LDL-C, and triglycerides, the effect shown (–5.26 mg/dL, –4.25 mg/dL, and –6.26 mg/dL, respectively) was less than that noted above when the soy protein was used in conjunction with other dietary interventions.47 In addition, the effects of isolated soy protein appear to be considerably less than those achieved by increasing soy food consumption in general. Much of the cholesterol-lowering effect of soy foods may relate more to the isoflavone and soluble fiber content rather than the protein. In an earlier meta-analysis based more on soy protein intake from other sources in addition to soy protein isolate, reductions were much more significant for total cholesterol (–23.2 mg/dL), LDL-C (–21.7 mg/dL), and triglycerides (–13.3 mg/dL) but still relatively modest from a clinical perspective.47a Although these results do support the recommendation to increase soy protein intake in the dietary approach to hypercholesterolemia, given the relatively small effect of soy protein on lipids, it is imperative that other dietary recommendations, such as reducing the dietary intake of saturated fat, trans fatty acids, and cholesterol as well as increasing the dietary intake of monounsaturated fats, soluble fiber, and nuts also be promoted.
Despite research documenting the benefits of nonpharmacologic approaches, it is unlikely that lowering LDL-C with statin drugs will be supplanted as the primary therapy in lipid management and the prevention of CAD at any time in the near future. In 2011, it was estimated that more than one of every six adults—nearly 40 million people—was taking a statin drug to lower LDL-C. Therefore, the focus with many patients will be on the support of statin therapy. For example, it appears that individuals taking statin drugs must supplement with coenzyme Q10 (CoQ10). For the synthesis of cholesterol, not only HMG-CoA reductase but also CoQ10 is required. Thus, administration of these drugs might compromise the status of CoQ10 by decreasing its synthesis. Even modest dosages of various statins have been shown to lower serum levels of CoQ10. Researchers have concluded that inhibition of CoQ10 synthesis by statin drugs could explain the most commonly reported adverse effects, especially fatigue and muscle pain, as well as more serious side effects such as rhabdomyolysis.48,49 CoQ10 supplementation in subjects on statin drugs has also been shown to reduce markers of oxidative damage.
Statins are also gaining popularity as a prescription method to lower CRP. In one study, a group of 186 individuals with type 2 diabetes was selected to receive 10 mg of atorvastatin (Lipitor), 80 mg of atorvastatin, or a placebo for 30 weeks. In those given placebo, CRP levels increased by 6.6%, decreased by 15% in the 10-mg group, and by 47% in the 80-mg group. In a study with pravastatin, 40 mg daily lowered CRP levels by 13%.50 Although many physicians appear to be aware of the effect of atorvastatin and pravastatin on CRP, they do not seem to be aware of the natural approaches to lowering CRP discussed below, including diet, niacin, vitamin E, and other measures.
The Importance of Soluble Dietary Fiber in Lowering Cholesterol
Chapter 52 provides a complete discussion of the benefits of dietary fiber. It is well established that the soluble dietary fiber found in legumes, fruit, and vegetables is effective in lowering cholesterol levels.51 The greater the degree of viscosity or gel-forming nature, the greater the effect of a particular dietary fiber has on lowering cholesterol levels. New, highly viscous, soluble fiber blends are showing greater effect than previously used fiber sources, leading to more reasonable dosage recommendations (the cholesterol-lowering effect of soluble fiber is clearly dose-dependent).52,53 Table 148-3 shows the average dosages and reductions noted in clinical trials with soluble fiber supplements.
TABLE 148-3 Impact of Various Sources of Fiber on Serum Cholesterol Levels
| FIBER | DOSAGE (G) | TYPICAL REDUCTION IN TOTAL CHOLESTEROL |
|---|---|---|
| Oat bran (dry) | 50-100 | 20% |
| Guar gum | 9-15 | 10% |
| Pectin | 6-10 | 5% |
| Psyllium | 10-20 | 10-20% |
| Vegetable fiber | 27 | 10% |
Many of the studies featured oat preparations containing either oat bran or oatmeal.54 The overwhelming majority of these studies demonstrated that individuals with high cholesterol levels see significant reductions with frequent oatmeal or oat bran consumption. In contrast, individuals with normal or low cholesterol levels see little change. In individuals with high cholesterol levels (above 200 mg/dL), the consumption of the equivalent of 3 g of soluble oat fiber typically lowers total cholesterol by 8% to 23%. This is highly significant, because with each 1% drop in serum cholesterol level there is a 2% decrease in the risk of developing heart disease. In approximately one bowl of ready-to-eat oat bran cereal or oatmeal are 3 g of fiber. Although oatmeal’s fiber content (7%) is less than that of oat bran (15% to 26%), it has been determined that the polyunsaturated fatty acids contribute as much to the cholesterol-lowering effects of oats as the fiber content. Although oat bran has a higher fiber content, oatmeal is higher in polyunsaturated fatty acids.
In an effort to lower cholesterol with dietary fiber, patients should be encouraged to eat 35 g of fiber daily from fiber-rich foods, a full listing of which can be found in Chapter 52. Achieving higher fiber intake is associated not only with lower cholesterol levels but also with lower inflammatory mediators like CRP.55
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree