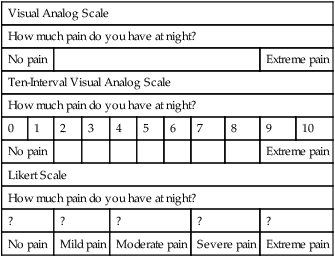
Client-centered care is a high priority in the health professions. The commonly used subjective, objective, assessment, and plan (SOAP) note method of documentation denotes the client’s subjective discussion of recovery as our first priority. The problem, however, is the effective intake of the client’s point of view and the consideration of this information from a quantitative and qualitative perspective. Quantitative aspects of evaluation and treatment include those variables that are measured in a standardized fashion and result in numeric data.1 Typical quantitative tools include goniometric measurement, manual muscle testing, and sensory evaluations. These tests often are referred to using terms such as objective or performance-based.2,3 Qualitative information, in comparison, is subjective and often consists of client narratives1 (Box 8-1). Symptoms, abilities, and participation in daily activities are typical examples of subjective information. For client-centered care providers, the challenge lies in attempting to measure and interpret qualitative information as a means of directing evaluation and treatment. Subjective information is considered to play a crucial role in maximizing therapeutic outcomes, and much research has been dedicated to this topic. Literature pertaining to client outcomes is littered with terms such as quality of life, emotional function, disability, and health status.4,5 Many researchers are pursuing measurement of these complex components in the form of client self-report outcome measures. These measures most often are found in questionnaire form using the visual analog scale (VAS) or Likert scale (Box 8-2). The development of these tools can be considered a conduit toward incorporating subjective data into evaluation and treatment planning and a means for analyzing and adding to the current evaluative repertoire. As therapists, our goal is to evaluate our clients accurately and consistently with tools that are designed to detect changes in clinical status. Research terms for these expectations include validity, reliability, and responsiveness. As a group, these variables are known as psychometric properties. Validity is the degree to which an instrument measures what it is intended to measure.1 An outcome measure can be defined specifically as having construct, criterion, and/or content (face) validity. Construct validity refers to a comparison between a new measure and an associated measure, criterion validity compares a new measure with a gold standard, and content validity considers accurate measurement of a specific domain.6 Reliability is the degree of consistency with which an instrument or rater measures a variable, and responsiveness is the ability of a test to demonstrate change.1 Responsiveness often is referred to as sensitivity to (clinical) change and is established if change in scores accurately represents change in clinical status.7 Similarities between measurement of responsiveness and validity have been addressed recently by researchers who question whether the two are distinct psychometric properties.8 Normative data, minimal detectable change, and minimally clinically important difference (MCID) are terms used to define analysis of client scores. Normative data can be considered as average scores for large groups of clients with similar diagnoses and abilities. As clinicians, we are familiar with norms in objective measures, such as grip and pinch strength. Normative values are helpful in providing a framework with which to compare client scores. With therapeutic progress or decline, we should expect to see a change in client-rated scores. The minimal detectable change is defined as a valid change in score that is not due to chance. MCID, in comparison, goes beyond valid change to assess meaningful difference in client function.9 The assessment of clinical outcomes from a qualitative and quantitative perspective is strongly encouraged in response to research findings.10 Clinical evaluation tools, such as range of motion, have been shown to demonstrate poor reliability11 and decreased responsiveness when compared with client self-report measures.12–14 Research also has shown a suboptimal relationship between client self-report of quality of life and ratings from health care providers.15 In addition, it is recommended that the client is the one who ultimately should assess the importance of change in health status.17 Despite these findings, incorporation of standardized, self-report outcome measures in treatment and/or clinical research is currently inconsistent.18 Over the past 25 years, client self-report outcome measures have been researched extensively and are readily available for use in clinical practice. These measures can be categorized as generic, regional, or disease-specific. Generic measures can be used to compare health conditions and therefore can assist in the analysis of policies and funds distribution. Regional measuresare designed to demonstrate changes at the systems level, whereas disease-specific measures are intended to be highly responsive for individual diagnoses.13 The purpose of this chapter is to introduce standardized, self-report outcome measures in the regional and disease-specific categories as a means of facilitating their incorporation into upper extremity evaluation and intervention. The Disabilities of the Arm, Shoulder, and Hand (DASH) index was a joint effort of the American Academy of Orthopaedic Surgeons (AAOS), American Society for Surgery of the Hand (ASSH), American Association for Hand Surgery (AAHS), the Council of Musculoskeletal Specialty Societies (COMSS), and the Institute for Work and Health (IWH).19 The DASH was conceptualized as a tool that would facilitate comparison of conditions throughout the upper extremity while considering it a single functional unit. Careful development of this tool, including an extensive literature review19,20 and consideration of questions and attribution,21 have made it a well-recognized and popular tool in upper extremity research and clinical practice. Thirteen existing outcome measures were reviewed to create a pool of more than 800 possible items. These items were reduced to 30 through a process of expert opinion and group selection.19,20 Two concepts of symptoms (9 items) and functional status (21 items) are addressed in the DASH with functional status being classified further into domains of physical, social, and psychological status. A Likert scale format is used for assessment; options range from 1 (no difficulty, symptoms, or limitations) to 5 (unable to complete activities and extreme symptomatology). The DASH is scored when at least 27 items have been completed using a simple equation offered by the authors.22 This equation anchors the score to a zero base; resultant scores range from 0 (no disability) to 100 (completely disabled). The DASH also includes optional work and sports/performing arts modules (4 items each).10 The DASH has been shown to correlate with both general health measures and joint-specific measures.23 A systematic review has been published stating that the DASH remains reliable and valid when translated into Armenian, Russian, Chinese, French, German, Swedish, Italian, Portuguese, and Greek.24 Normative data, including mean scores and standard deviations, have been published by the AAOS.25 Psychometric properties of the DASH have been researched thoroughly. Studies in upper extremity clinics including a variety of diagnoses have demonstrated validity, excellent reliability, and responsiveness to clinical change.22,26–29 The DASH also has been established as possessing the following attributes: content validity for clients with psoriatic arthritis;30 construct validity for clients with ulnar wrist disorders,31 carpal tunnel syndrome, and subacromial impingement;32 reliability for clients with pathologic conditions of the elbow18,33 and musculoskeletal complaints from textile workers;34 convergent and divergent validity for clients following arthroplasty of the CMC joint;35 and test-retest reliability, concurrent, convergent, and face validity for patients with rheumatoid arthritis.36 Responsiveness/sensitivity to clinical change has been recognized for the DASH when used with clients following carpal tunnel release,37–39 distal radius fracture,40,41 wrist pain,39 humeral fractures,42 rotator cuff repairs,43,44 shoulder instability,45 rheumatoid arthritis,46,47 and various shoulder pathology.48 In addition, the DASH has been used to determine outcomes for clients with distal humeral fractures,49,50 radial nerve palsy,51 and acute traumatic hand injuries.52 The DASH was found to be the best instrument for evaluating patients with disorders involving multiple joints in the upper limb53,54 and has been suggested as the most widely tested instrument.55 The content of the DASH has been found to link well with the International Classification of the Functioning, Disability, and Health (ICF) framework56,57 and has been reported as a potential patient-centered tool to aid in comprehensive care.58,59 On the other hand, the DASH has been demonstrated to be comparable to, but slightly less responsive than, the Canadian Occupational Performance Measure (COPM),26 the Patient-Rated Wrist/Hand Evaluation (PRWHE),28 the Boston Questionnaire,37,38 the Western Ontario Shoulder Instability Index (WOSI),45,60 and the Shoulder Pain and Disability Index (SPADI).61 The DASH was found to be less sensitive to differences in functional outcomes between clients with hand injuries62 and clients following wrist arthroplasty or arthrodesis.63 In addition, a Rasch analysis questioned the validity of the DASH when utilized for patients with multiple sclerosis.64 The QuickDASH was developed by Dr. Borcas Beaton and colleagues at the IWH in 2005. The purpose of creating the QuickDASH was to provide a valid and reliable shortened version of the full DASH that would be easier to use in a clinical context for both the patient and clinician/researcher.65 The full DASH consists of 16 domains, but for the purposes of the QuickDASH, only 11 domains were utilized; therefore reducing the number of items for the assessment to 11. Three item-reduction techniques were utilized to analyze the 30 item full DASH to determine which 11 items would be clinically sensible to include in the QuickDASH.65 The QuickDASH consists of 11 items addressing the symptoms and functionality of people with any or multiple disorders of the upper extremity. The QuickDASH is similarly formatted to the full DASH using a Likert scale with the options ranging from 1 (no difficulty, symptoms, or limitations) to 5 (unable to complete activities and extreme symptomatology). The QuickDASH can be scored when 10 of the 11 items have been completed; allowing only one “missed” item. The scoring equation used to calculate the full DASH is also used to score the QuickDASH with scores ranging from 0 (no disability) to 100 (completely disabled).65 The QuickDASH has been translated into the Turkish language and deemed as reliable and valid as the original tool for patients with carpal tunnel syndrome.66 The QuickDASH was originally found to have reliability, construct validity, and responsiveness to clinical change when used with 200 patients with either proximal or distal disorders of the upper extremity.65 The QuickDASH and full DASH were found to have similar responsiveness to clinical change for patients with hand trauma and degenerative hand conditions.67 The QuickDASH was significantly correlated with changes of ROM and can be utilized to detect clinical change in children with supracondylar humeral fractures.68 The QuickDASH is an adequate tool for a summary assessment of symptoms and functionality, but the full DASH should is suggested for differentiating assessment of symptoms and function for patients following shoulder and carpometacarpal arthroplasty.69 The cross-sectional and test-retest reliability of the full DASH and the QuickDASH are similar, insinuating that the QuickDASH can be used in clinical practice instead of the full-length DASH.70 The Michigan Hand Outcomes Questionnaire (MHQ) was developed by Dr. Kevin Chung and colleagues71 at the University of Michigan in 1998. The MHQ is defined as a hand-specific questionnaire; the authors specifically address function of each upper extremity separately as a means of analyzing independent use, hand dominance, and bilateral involvement.10 Previously established tools and client input were used to create the MHQ.71 The MHQ consists of 67 questions including six domains, demographics, and work history. Domains of overall hand function, physical function with activities of daily living tasks, esthetics, and satisfaction with hand function are answered specific to each hand. Responses in the domains of pain and work performance are given regarding both hands. All domain items are formatted in Likert scales ranging from 1 to 5 with lower scores indicating higher function in all domains except pain. If less than 50% of a scale is complete, the scale is not scored, and if more than two scales are incomplete, a final score cannot be calculated. Raw scores are summed for each hand; bilateral scores are calculated by averaging scores from both hands. Summed and averaged scores are normalized from 0 to 100 according to the scoring algorithm as found in the original publication.71 Scores closer to 100 on all scales indicate increased function and decreased pain. The MCIDs for the MHQ have been published specifically for patients with carpal tunnel syndrome (pain: 23, function: 13, work: 8) and rheumatoid arthritis (pain: 11, function: 13).72 The MHQ was originally found to be reliable and valid in a study including 200 clients with various upper extremity diagnoses.71 Responsiveness as a function of significant clinical change was noted in a similar, diverse client group.7 In addition, the MHQ has been found to be sensitive to clinical change for patients with carpal tunnel syndrome,39,55 carpal tunnel release,73–76 distal radius fractures,77 wrist pain, and finger contracture.39 The MHQ has been established as valid and reliable for clients with rheumatoid arthritis,78,79 osteoarthritis,80 and diabetes.81 The MHQ has been suggested to be reliable and valid for patients with finger and wrist disorders but less so for nerve disorders.82 Multiple sources have disputed which functional outcome assessment, MHQ or DASH, is considered to be most responsive to clinical change.62,83,84 The MHQ has been correlated to grip strength85 and a standardized test of activities of daily living function,86 and it was reported to be sensitive to change in a long-term follow-up study of clients with metacarpophalangeal joint arthroplasties.87 Most recently, a brief, 12-item version of the MHQ has been created and was reported to maintain the same psychometric properties as the original MHQ.88 The Patient Evaluation Measure (PEM) was introduced by Macey and Burke89 in 1995. Developed in the United Kingdom, the PEM is novel in that it addresses client satisfaction as a primary component of outcome measurement. The original PEM included three parts: treatment (5 items), “how your hand is now” (10 items), and overall assessment (3 items). A second version was published in 2001 that titled the second section as the Hand Health Questionnaire and added a question to this section addressing duration of pain. The PEM is presented in a seven-interval VAS with lower scores indicating increased satisfaction and function. Parts two and three are summed and calculated as a percentage of the possible score. Part one is not included in the scoring process, and the authors suggest that clients should be given their previous answers when repeating this measure.90 A cultural difference in language is noted in the use of the activity example “fiddly things,” which is clarified as fine dextrous tasks.91 The PEM was found to be reliable, highly valid, and highly responsive in patients with carpal tunnel syndrome76 and a study of 80 clients with scaphoid fractures.90 Research including 35 clients with multiple diagnoses demonstrated very good reliability and validity as correlated with grip strength.91 The PEM was also used for outcomes measurement in a follow-up study of clients with palmar wrist ganglia92 and found to be valid when assessing the outcomes of patients with distal radius fractures.93 The PEM was compared to the DASH and MHQ and found to be the easiest to use according to 100 randomly selected patients with varying hand and wrist disorders.82,94 In addition, the PEM was found to be more responsive to change than the DASH when used as an outcome measure for patients with carpal tunnel syndrome.94 Dr. Joy MacDermid and colleagues in Ontario, Canada, have carefully researched and developed four client-rated tools for outcome evaluation: the Patient-Rated Wrist Evaluation (PRWE), the Patient-Rated Tennis Elbow Evaluation (PRTEE), the Patient-Rated Elbow Evaluation (PREE), and the Patient-Rated Wrist/Hand Evaluation (PRWHE). The PRWE was the first measure of the four to be developed; the process included a survey of the International Wrist Investigators for content and format. The goal in creating the PRWE was to capture client-rated measurement of impairment, disability, and handicap that could be used in clinical and research settings.3 The three remaining tools were published as adaptations to the PRWE that more specifically could address measurement of outcomes for clients with lateral epicondylitis (PRTEE), pathologic conditions of the elbow (PREE), and hand injuries (PRWHE). All four tools include two scales of pain and function. The pain scales include 5 to 6 items that address magnitude and frequency of pain. Activities that might precipitate pain are specific to each tool. Function scales are divided further to specific and usual activities. Usual activities are identical for each tool and include personal care, household management, work, and recreation. Specific activities on PRWE, PRWHE, and PRTEE consist of 10 items that pertain to the anatomic area under consideration. In comparison, the PREE lists 15 specific activities. Responses are marked on a scale ranging from 0 (no pain/difficulty) to 10 (worst pain imaginable/unable to do) as perceived over the past week. The authors present multiple scoring options; scales can be summed individually or combined with simple calculations to yield a total score of up to 100 points.3,18 Higher scores on all scales indicate increased pain and decreased function. The PRWE was published in 1996.3 Reliability and content, construct, and criterion validity have been established for this tool.3,95 These properties were reinforced in clients with distal radius and scaphoid fractures, and the PRWE total score was found to demonstrate greater reliability than individual subscale scores.96,97 Responsiveness of the PRWE was determined in a prospective study of 275 clients with distal radius fractures; this publication also offers means and standard deviations for the tool.98 The PRWE was more responsive than the DASH and SF-36 for clients following distal radius fracture.40,53,98 The PRWE was not sensitive to differences in outcomes between clients with wrist arthroplasty or arthrodesis63 and was less sensitive than grip strength testing for clients with unstable wrist fractures.99 The PRWE was chosen as an outcome measure in a publication that advocates low-level heat wraps for wrist pain,100 and translations into Japanese,101 Hindi,102 and Swedish languages103,104 have been considered as valid and reliable as the original tool. The PREE was introduced in 2001 in a study that articulated excellent reliability for the total score, high reliability for the pain subscale, and moderate to high reliability for the function subscale.18 The PREE has successfully been translated in to a German version suggested to be as reliable and valid as the original tool.105 The PRWHE differs from the PRWE only in introductory wording, changing “wrist” to “wrist/hand,” and the addition of one appearance question. The PRWHE was found to be more responsive than the DASH in a study of 60 clients with a variety of hand injuries; however, the appearance question was less responsive than comparable scales.28 Furthermore, according to a systematic review of functional outcome assessments, the PRWHE was found to have good construct validity and responsiveness for patients with varying wrist injuries, which was considered to be marginally superior than the DASH.55 The PRWHE was also found to be a valid tool when assessing pain and/or disability following arthroplasty of the carpometacarpal joint in the osteoarthritic hand.106 The American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment form was developed by the Research Committee for American Shoulder and Elbow Surgeons in 1993 and published in 1994.107 A later publication excluded five questions and subsequent studies of psychometric properties are based off of this modified edition.108 The ASES was designed to be a baseline measurement of shoulder and elbow function that would be applicable to any diagnosis involving these anatomical structures.107 The ASES consists of two sections: pain and activities of daily living (ADLs). The pain section is rated on a VAS ranging from 0 (no pain at all) to 10 (pain as bad as it can be).107 The ADL section assesses ten ADLs on a four point ordinal scale ranging from 0 (unable to do the activity) to 3 (no difficulty). Each upper extremity is assessed separately and scored according to the equation provided by the authors.107,108 The ASES was found to have test-retest reliability; construct and discriminant validity; and responsiveness to clinical change for patients with various shoulder pathologies; and a MCID of 6.4.108–111 It was suggested to demonstrate reliability and construct validity for patients following shoulder joint operations,112 and reliability and responsiveness following shoulder surgery.113,114 In addition, the ASES was found to have reliability; criterion and construct validity; and responsiveness to clinical change for 455 patients with shoulder instability, rotator cuff disease, and shoulder arthritis.115 The Shoulder Pain and Disability Index (SPADI) was designed as a regional measure aimed at capturing patient self-reported pain and disability about the shoulder. Originally presented as VAS,116 the more commonly used second version includes a numerical rating scale for ease in administration and scoring.117 The SPADI includes two domains: a five-item pain subscale and an eight-item disability subscale. Patients are allowed to mark one item as not applicable that is then omitted from scoring. In cases with more than two not applicable prompts, no score is calculated. Summation and transformation of subscales yields a score out of 100; averaging the pain and disability scores then creates a final score. A higher score on the SPADI is indicative of higher perceived pain and/or disability. The MCID for this tool has been reported to range between 8 and 13 points.61 The SPADI has been validated in Turkish,118 German,119 Portugese,120,121 and Norwegian.122 Excellent reliability has been consistently reported for this tool61,95 with high internal consistency suggested for the pain and disability subscales in patients with chronic shoulder symptoms.123,124 The SPADI was found to be significantly more responsive than: the Western Ontario Rotator Cuff Index (WORC) and Oxford Shoulder Scale for patients with rotator cuff disease;125 the Croft Index and DASH for patients with adhesive capsulitis;126 the Sickness Impact Profile for patients with shoulder pain;127 and the DASH for patients following total shoulder arthroplasties.128 Research suggests a strong negative correlation between the disability subscale scores and shoulder range of motion and a positive correlation between the disability subscale scores and age.124 Higher pain and disability have also been correlated with passive or negative coping strategies.123 Limitations of the SPADI include its identification as unidimensional for patients with adhesive capsulitis129 and its limited attention to occupational/recreational disability.127 Table 8-1 provides information on the aforementioned regional measures. TABLE 8-1
Assessment of Functional Outcomes
Regional Measures
Disabilities of the Arm, Shoulder, and Hand Index
QuickDASH
Michigan Hand Outcomes Questionnaire
Patient Evaluation Measure
Patient-Rated Evaluation Methods
American Shoulder and Elbow Surgeons Standardized Shoulder Assessment
The Shoulder Pain and Disability Index
Title
Date and Location
Questions
Format
To Obtain
DASH
1996, AAOS, ASSH, AASH, COMSS, and IWH
Symptoms: 9
Functional status: 21
Total: 30
Optional—Work: 4
Sports/performing arts: 4
Likert scale
www.dash.iwh.on.ca
QuickDASH
2005, IWH
Symptoms: 3
Functional: 8
Total: 11
Likert scale
www.dash.iwh.on.ca/quickdash
MHQ
1998, University of Michigan
Total: 67
Hand function, physical function with ADL, esthetics, satisfaction with hand function, demographics, and work history
Likert scale
www.med.umich.edu/surgery/plastic/research/department/studies/mi_hand_outcome.shtml
PEM
1995, United Kingdom
Treatment: 5
Hand Health
Questionnaire: 11
Overall assessment: 3
7-interval VAS
Dias et al90
PRWE
1996, Ontario
Pain: 5
Function: 10
Total: 15
10-interval VAS
Dr. Joy MacDermid
Hand and Upper Limb Centre
St. Joseph’s Health Centre
268 Grosvenor Street
London, Ontario N6A 3AB
PREE
2001, Ontario
Pain: 5
Function: 15
Total: 20
10-interval VAS
Dr. Joy MacDermid
Hand and Upper Limb Centre
St. Joseph’s Health Centre
268 Grosvenor Street
London, Ontario N6A 3AB
PRWHE
2004, Ontario
Pain: 5
Function: 10
Total: 15
Optional—
Appearance: 1
10-interval VAS
Dr. Joy MacDermid
Hand and Upper Limb Centre
St. Joseph’s Health Centre
268 Grosvenor Street
London, Ontario N6A 3AB
ASES
Standardized Shoulder Assessment Form
1993, Research Committee for American Shoulder and Elbow Surgeons
Pain: 1
Function: 10
Total: 11
Pain: VAS
Function: Likert scale
Michener LA, McClure PW, Sennett BJ108
SPADI
Total: 13
1991
Roach KE, Budiman-Mak E, Songsiridej N, et al116
Williams JW Jr., Holleman DR Jr., Simel DL117
Pain: 5
Disability: 8
Likert scale
www.workcover.com/public/download.aspx?id=799 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Assessment of Functional Outcomes