Fig. 23.1
Traumatic full-thickness chondral lesion of the medial femoral condyle
PCL insufficiency has known detrimental effects on joint mechanics of the knee, and the kinematics can be altered dramatically depending on the individual’s activities [19]. Sectioning of the PCL increases both patellofemoral and medial compartment contact pressures [20, 21]. The magnitude and location of peak articular cartilage contact pressures during knee flexion are altered with PCL deficiency, both increasing absolute peak loads and shifting contact pressures to a more anterior and medial location within the medial compartment [22]. These altered kinematics help to explain the more typical locations of the resulting symptoms and degenerative changes that may result with PCL insufficiency over time, with the medial femoral condyle being the most likely (31–78 %) affected region followed by the patellofemoral compartment (23–47 %) [14, 23].
Following a severe multiligament injury, the development of progressive degenerative changes is often ubiquitous with time [15–18]. This degenerative course is felt to be multifactorial, and despite the high rate of articular degeneration that occurs following these injuries, there is no current evidence to suggest that a focal, acute chondral injury will become symptomatic or be the primary cause of progressive joint degeneration [24]. Degenerative changes following ligamentous knee injury are influenced by many factors including altered joint kinematics, persistent instability, meniscus integrity, weight, body mass index, as well as macroscopic- and cellular level articular cartilage damage [18, 25–30]. Many of these patients may ultimately require an arthroplasty to address their advanced posttraumatic arthritis [31].
With chronic isolated PCL instability, patients often also go on to develop articular degeneration with time, although progression rate may be variable [23, 32–34]. Similar to the multiligament situation, factors leading to the degenerative course are likely multifactorial and it is currently often unknown the contribution an acute chondral lesion will make to this degenerative progression. The natural history of most acute chondral injuries is poorly understood, and it is often unknown whether the treatment will even alter the natural history, especially in a joint with abnormal kinematics. Many chondral lesions will remain asymptomatic for a period of time without any treatment. Others may be quite debilitating and progress with time. Determining which course an acute lesion will take in the setting of an acute PCL or multiligamentous injury is realistically impossible at this time.
With limited evidence that procedures addressing the articular damage will necessarily alter the overall natural history of the joint, it is important to evaluate the lesion, joint, and patient characteristics to determine optimal treatment. Given the spectrum of cartilage lesions that may be encountered, different treatment options can be considered to address a specific lesion. Inherent to the discussion of articular cartilage surgery is the reality that optimal treatment is often unknown and controversial, thus individual surgeon preferences and personal algorithms often play a significant role in treatment.
Cartilage restorative options are more limited in the acute multiligament injury setting since surgical intervention is often recommended within 3 weeks of the injury [35]. It is reasonable to treat the majority of high-grade lesions with low-morbidity, expeditious, available interventions in the acute setting [28]. For the subset of lesions that cause persistent symptoms despite primary acute treatment, secondary articular-cartilage resurfacing procedures are performed according to accepted algorithms. Many lesions may never require further treatment; however, degenerative changes often occur in patients over time. When contemplating treatment of a more chronic articular-cartilage lesion, it is important to appreciate the potential contributing factors to the development of the lesion. Some of these issues may be able to be addressed, while others may not. Realizing despite our best efforts, this can be a very challenging patient population. The goal of the articular cartilage surgeon is to address symptomatic lesions in order to improve pain and function, with the hope of delaying the need for future arthroplasty. Realistically, however, with time many patients with isolated PCL injury and most with severe multiligamentous injury will eventually require arthroplasty to address degenerative changes that often progress with time.
Treatment of Acute Articular-Cartilage Lesions Associated with PCL Injury
With limited evidence to support definitive recommendations for addressing articular defects occurring with a concomitant ligamentous injury in the acute setting, there is considerable debate among orthopedists regarding the most appropriate surgical treatment for an incidental and potentially asymptomatic lesion versus what may be a potentially symptomatic defect [24, 36–38]. In the acute setting, it may be impossible to determine if a chondral lesion is or will remain symptomatic. While many patients with PCL insufficiency will go on to develop chronic degenerative changes over time, it is truly unknown the specific contribution an acute defect will make, versus other factors, to this frequent ultimate outcome [4, 15, 23–26, 32, 36, 39]. When considering data on acute anterior cruciate ligament (ACL) injury, patients with an acute high-grade articular cartilage defect left untreated were shown to only have slightly inferior outcomes compared to patients without chondral lesions 15 years following ACL reconstruction [24, 36]. Other studies, however, report that these focal lesions can cause significant morbidity [29, 38, 40]. At this time, we feel that it is reasonable to treat a high-grade lesion in the acute setting in a patient who is undergoing surgery for another primary indication. However, the authors favor expeditious, less-invasive techniques, which minimize morbidity when treating chondral lesions in this acute treatment group.
Perhaps in the future, we will be able to better determine if “more invasive” cartilage procedures or even acute treatment at all will change the natural history of the single or multiligament-injured knee. Currently, there are no trials that investigate the effect of treatment versus no treatment. Additionally, there are not many comparison studies among treatments in not only the PCL or multiligament-injury group but also in the much larger isolated ACL reconstruction population. Several investigators have published case series of combined ACL reconstruction and osteochondral autograft transfer, microfracture, or autologous chondrocyte implantation (ACI) with reasonable short-term outcomes [11, 29]. One recent study did show higher subjective outcome scores with osteochondral autograft transfer when compared to microfracture in an ACL reconstruction population at 3-year follow-up [41]. Other studies, which have focused more on symptomatic lesions, have more variable results. Some show superiority or perhaps greater durability of more invasive procedures like osteochondral autologous transfer system (OATS) or ACI, while others do not [41–43]. There does seem to be a trend towards inferiority of microfracture relative to hyaline-resurfacing options in terms of durability with time; however, this is still a topic of debate [44–46].
Although it makes sense to try and resurface a high-grade defect based on our experience with treating symptomatic defects, the results of any cartilage restoration procedure for an acute traumatic or incidental lesion may have optimal results relative to other cohorts. However, since certainly some patients with higher-grade lesions do go on to become symptomatic in our and others’ experience, treatment over nontreatment is reasonable in this setting [11, 29, 40]. An acute treatment algorithm is proposed based on available options yet attempting to minimize morbidity when treating full-thickness defects in an often young individual (Fig. 23.2). The senior author (KFB) acknowledges that this is based on anecdotal experience, and there is no good evidence to support one treatment method over another in an acute and potentially asymptomatic lesion [11, 40].
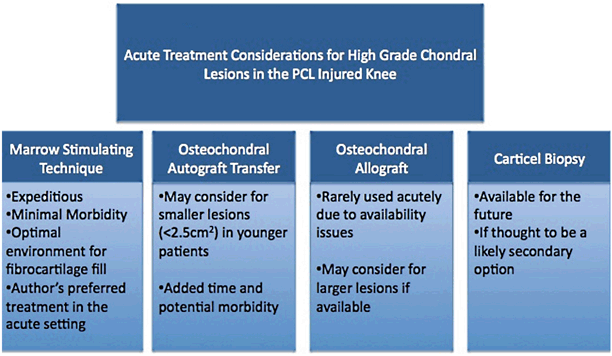

Fig. 23.2
Acute treatment algorithm for a high-grade chondral lesion in the PCL or multiligament-injured knee. PCL posterior cruciate ligament. (With kind permission from Springer Science + Business Media: The Multiple Ligament Injured Knee [31])
Debridement/Chondroplasty
Arthroscopic chondroplasty is the treatment of choice whenever a partial-thickness (ICRS Grade 2) articular cartilage lesion is present with unstable edges or fragments. The senior author routinely will use this simple procedure for higher-grade (ICRS 3a) lesions if there is still a layer of articular cartilage present over the calcified cartilage layer. If this layer has been violated and calcified cartilage is visible (ICRS Grade 3b or Outerbridge Grade 4), other treatment options are preferred, especially in younger, more active populations.
Chondroplasty is used to help alleviate mechanical symptoms that may be caused by loose, unstable fragments of cartilage. Removing only the unstable cartilage gives the surgeon a quick, low-morbidity treatment option to address a chondral lesion when reconstructing the PCL or multiligament knee in the acute setting. Having an expeditious treatment option is beneficial as the reconstruction itself can be a time-consuming procedure. Debridement of the cartilage is done with either a mechanical shaver or a more modern radiofrequency-type device, and there is some debate over which is the better option. Cell death from thermal injury has been cited as a concern with radiofrequency devices although this is controversial [29, 47].
Microfracture
Marrow-stimulating techniques, such as microfracture, are often used in an attempt to induce the formation of primarily fibrocartilage repair tissue within a high-grade to full-thickness defect. Lesion edges are debrided to a stable rim followed by penetration of the subchondral plate by either an awl-type impaction device by drilling utilizing a small bit. These small subchondral access holes, which should be separated by 3–5 mm, serve as a conduit for blood components into the defect site (Fig. 23.3). The goal is to create a stable fibrin clot within the defect, which will remain to support the formation of repair tissue. Marrow-derived fibrin clot contains mesenchymal stem cells, which can differentiate into fibrochondrocytes. These cells produce a fibrocartilage repair tissue that contains type I and type II collagen [48, 49]. Microfracture has been shown to produce variable fibrocartilage defect fill, based off of short-term magnetic resonance imaging (MRI) data [50–52]. While this repair tissue has inferior biomechanical properties and wear characteristics compared to hyaline cartilage, there is a correlation, at least in the short term, between the amount of fibrocartilage repair tissue found on MRI and patient outcomes [49–53].
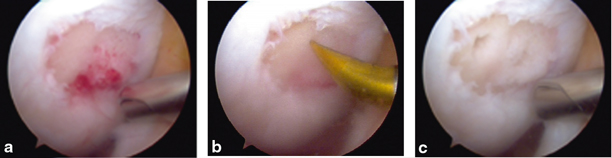

Fig. 23.3
a Traumatic full-thickness chondral lesion of the femoral condyle sustained during a knee dislocation. b and c Microfracture of the relatively small lesion performed during acute reconstruction
Microfracture is generally considered a good first-line treatment option for many acute full-thickness lesions, especially lesions of the femoral condyle [54]. The procedure is fairly straightforward, quick, and very cost-effective. Again, a PCL or multiligament reconstruction is often a time-demanding procedure, and any treatment that can address the chondral lesion expeditiously while minimizing the patient’s morbidity should be considered. Microfracture is currently the senior author’s most common treatment of choice for full-thickness lesions in the acute PCL/multiligament-injured knee as the treatment time and the morbidity of the procedure is minimized.
Clinical outcome studies of microfracture report an improvement of symptoms in 50–90% of patients [29, 49, 50, 55–58]. These results vary, however, based on the characteristics of the lesion as well as the patient’s demographics, activity level, and duration of symptoms [55–59]. Patients tend to do worse with lesions > 2 cm2, age > 35 years, higher body mass index, patellofemoral lesions (particularly patella lesions), and symptomatology > 1 year [50, 55, 56, 59]. While microfracture has been shown to improve patients’ function in the short term, limited data is available on the long-term results following the procedures [55]. Available long-term data show that microfracture improves International Knee Documentation Committee (IKDC), Lysholm, and Tegner scores in the short term. These improvements, however, diminish over time, and treatment failures and degenerative arthritis can be expected in a significant percentage of patients, especially with larger or multiple lesions in older and more active patients [44–46, 54]. However, this may occur with any treatment in this often complex patient population.
Although controversial, in younger patients with symptomatic defects, there may be some downside to microfracturing a lesion when considering future treatment options. Higher-volume ACI surgeons have shown evidence that prior microfracture may adversely affect the outcomes of a secondary ACI procedure [60–62]. This is thought to likely be due to the formation of intralesional osteophytes, which form as a result of disruption of the subchondral plate. Authors who feel ACI may produce a more reliable and durable repair tissue may not microfracture a lesion for this reason. Secondary treatment options utilizing osteochondral grafts should not be affected since the osteochondral unit is replaced in this setting.
Osteochondral Autograft Transfer
Osteochondral autograft transfer/mosaicplasty transfers one or more osteoarticular cylindrical plugs from a lower weight-bearing area of the knee to a symptomatic location [63–65]. This technique has been utilized since the mid-1990s with some variation [63–65]. Some authors have utilized multiple, smaller-diameter plug “mosaic” configurations versus currently, many surgeons prefer to transfer fewer, larger-diameter plugs (Fig. 23.4). This is a good primary or secondary option for symptomatic chondral or osteochondral lesions less than 2.5 cm2. Delivering the autologous osteochondral plug allows reliable bone-to-bone healing with viable hyaline cartilage usually within 6 weeks of the procedure.
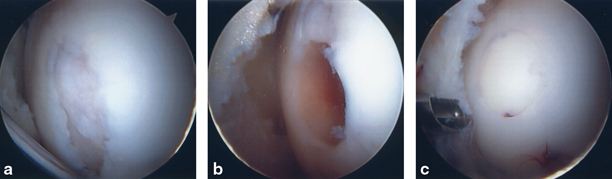

Fig. 23.4
a Full-thickness defect of the medial femoral condyle (MFC). b First recipient site prior to OAT implantation. c MFC defect following implantation of two OAT plugs overlapped in a “Mastercard” configuration. OAT osteochondral autograft transfer. (With kind permission from Springer Science + Business Media: The Multiple Ligament Injured Knee [31])
Contact stress studies have been used to help define optimal donor-site locations; however, results and expert opinion vary on the three recognized acceptable harvest-site locations within the knee [63–66]. Potential donor-site morbidity is a concern with this procedure. Hangody, who has the most extensive experience with the procedure, reports a 3–5 % incidence of morbidity [67]; however, there are variable rates of reported donor-site morbidity in the literature, and some studies report no donor-site morbidity [68–75]. Bioabsorbable scaffolds and allograft plugs have been used to backfill the donor sites to decrease postoperative hemarthrosis and donor-site morbidity [63]; however, it is unknown if donor-site morbidity is decreased in the long term, and case reports have shown that foreign body reactions can occur following the use of these scaffolds [76, 77]. One large center with probably the greatest experience has published studies revealing remodeling and tissue ingrowth occurs reliably over 2 years but with often poor MRI characteristics prior to that time, while others have not demonstrated synthetic grafts have led to increased bone ingrowth or osteoconductivity [78, 79].
Delivery of the osteochondral plugs can be technically challenging, and placing the graft perpendicular into the recipient’s site is critical to the success of the procedure. While this can be performed arthroscopically, surgeons often feel more comfortable using a limited arthrotomy to more reliably deliver plugs perpendicular to the articular surface. Similarly, the superolateral trochlea, which is likely the most popular donor-site location, is often accessed using a small lateral arthrotomy to obtain reproducibly perpendicular donor plugs (Fig. 23.5).
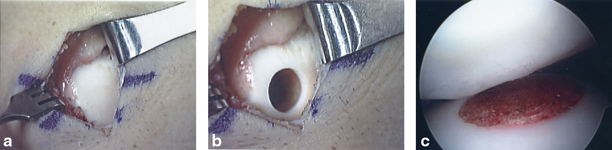

Fig. 23.5
a and b Superior lateral trochlea visualized thru mini-arthrotomy to harvest OAT donor plug. c Donor site (which was backfilled) does articulate under patella. OAT osteochondral autograft transfer. (With kind permission from Springer Science + Business Media: The Multiple Ligament Injured Knee [31])
Osteochondral autograft transfer, for a surgeon who is comfortable with the technique, is an option in the acute setting, and should be considered for a younger, active patient with a smaller lesion (< 2.5 cm2). However, given the technical difficulty of the procedure, additional surgical time should be expected, and further morbidity to the joint may occur. It is uncertain at this point if the increased procedural time and trauma make this the most optimal treatment choice in the acute setting, but it is clearly a viable option.
Fresh Osteochondral Allografts
Mutliligament Grade III PCL injuries addressed in the acute setting are often treated within 3 weeks, making it often difficult to obtain a fresh allograft within this limited time frame. Fresh osteochondral allografts are often used to treat larger symptomatic chondral or osteochondral lesions, and the procedure is most often used for the secondary treatment of symptomatic defects. However, if an allograft can be obtained in the acute setting, it may be a reasonable option for certain lesions. Depending on the nature of the lesion, it is often not necessary to obtain a perfect size match for a condylar lesion. We will not uncommonly request a condyle the same size or larger in order to increase the likelihood of obtaining a graft in a timely fashion.
ACI Biopsy
ACI (Carticel; Genzyme Corp, Cambridge, MA) is a two-staged procedure that harvests cartilage during the index procedure and implants cultured cells 4–6 weeks later [61, 80]. ACI is typically used to address persistently symptomatic lesions that have failed primary treatment, but if the surgeon feels that the lesion has a high probability of becoming persistently symptomatic, the index operation to procure the cartilage can be performed in the acute setting. This may be considered in larger-sized defects in a younger, more active patient.
The index biopsy procedure can be done quickly in the acute setting with the goal of minimizing morbidity, and performing the biopsy in the appropriate setting can avoid an additional biopsy procedure in the future. Cartilage is often procured from the lateral side of the intercondylar notch using curettes, and the specimen is sent to the Genzyme Corporation (Cambridge, MA) where the cells are isolated, cultured, and expanded in vitro for use in the second procedure (Fig. 23.6).
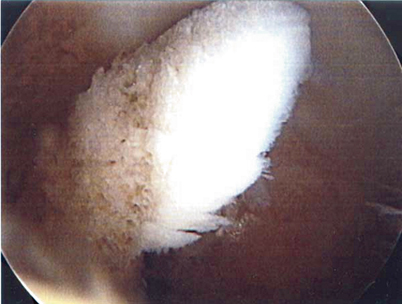

Fig. 23.6
Cartilage biopsy may be obtained at the index procedure if the surgeon feels it may be a future option. (With kind permission from Springer Science + Business Media: The Multiple Ligament Injured Knee [31])
Secondary Treatment for Symptomatic Articular Cartilage Lesions Associated with a PCL Injury
Similar to other articular cartilage treatment algorithms, patient and lesion factors need to be carefully considered when selecting the most appropriate articular cartilage treatment option in the setting of a persistently symptomatic lesion [81, 82]. Patient age, lesion size and location, activity level, and mechanical environment of the involved compartment(s) are factors, which will influence the treatment for these patients [28, 32, 83]. Due to the complexity of many of these patients, it can sometimes be quite difficult to assess the contribution of symptoms resulting from the chondral pathology versus the sequela of the overall joint trauma and altered kinematics, which likely significantly affects the articular cartilage. It is very important, not only in this group but also when treating all patients with articular cartilage pathology with a non-arthroplasty biologic procedure, for the patient and surgeon to have realistic outcome expectations. The goal in the younger patient populations is to significantly improve symptoms and postpone the need for an arthroplasty. However, many of these patients will still have a component of pain and functional disability [28, 32, 61, 83]. Middle-aged or certainly older patients may better be served with nonoperative treatment until their symptoms warrant an arthroplasty procedure.
Following recovery from initial treatment including prior ligament reconstruction, patients can be thoughtfully assessed in the office. In addition to an assessment of current complaints, a careful physical exam is essential to ascertain if the patient’s complaints and exam correlate to the chondral injury in question. Prior operative reports and arthroscopic pictures are very valuable as well. MRI with cartilage sequences may or may not be helpful depending on the time interval from the initial surgery and the clarity of the problem. Long-alignment films may be required if malalignment is suspected in the involved compartment. Diagnostic intra-articular injections are sometimes useful to differentiate between intra-articular and extra-articular sources of pain in the complicated patient. Unloader braces are occasionally utilized to assist in differentiating pain emanating from the medial tibiofemoral compartment versus other potential etiologies such as pain radiating from the patellofemoral compartment.
The following section of the chapter discusses potential options for the treatment of persistently symptomatic defects associated with a previous PCL or multiligament-injured knee. Special considerations for treatment of symptomatic chondral lesions in this patient population are highlighted in Fig. 23.7. This assumes that malalignment will be concomitantly corrected or was previously corrected. The more diffuse the chondrosis in the involved compartment, the more likely the author favors correcting the malalignment through an unloading osteotomy only. The more focal the defect, the more we tend to favor unloading the compartment and resurfacing the lesion at the same setting. If meniscal deficiency is thought to be a contributing factor, this should also be addressed at the same setting of the chondral resurfacing [84].
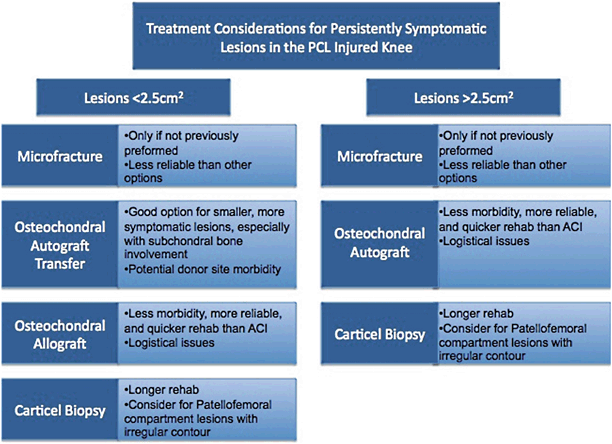

Fig. 23.7
Treatment options and considerations for persistently symptomatic lesions associated with the PCL or multiligament-injured knee. PCL posterior cruciate ligament (With kind permission from Springer Science + Business Media: The Multiple Ligament Injured Knee [31])
The younger the patient, the more aggressive we tend to be with biologic alternatives. The opposite is true with individuals who are older and more sedentary or if their pathology is beyond the scope of what can be reasonably addressed with a biologic approach. Unfortunately, many of these patients may be quite young for an arthroplasty, but it still may be their most reliable option when their symptoms justify further intervention.
Microfracture
Microfracture may be considered a viable treatment alternative if the lesion was initially untreated or simply debrided. The results of microfracture are generally considered to be worse with larger defects and patellofemoral lesions, especially in individuals over the age of 35 years. Also, the rate of return to sports when a symptomatic defect is treated may not be as high as with alternative treatment options [41, 58, 85, 86]. In the setting of an individual who has persistent symptoms, thought to be localized to a chondral lesion, in a previous isolated PCL injured or multiligament-injured knee, we tend to opt for other resurfacing alternatives, which may be more reliable or durable. However, microfracture is certainly a reasonable option for certain lesions previously untreated.
Osteochondral Autograft Transfer
OAT procedures have been used with success in the treatment of select chondral defects as outlined previously in this chapter. Advantages include the ability to resurface a defect with autologous viable hyaline cartilage utilizing locally available osteochondral grafts. The dowel grafts are press-fit and heal relatively quickly due to autologous bone-to-bone healing. Recent studies reveal osteochondral autograft transfer outcomes, may be favorable in comparison to microfracture in the athletic population [41, 42, 87]. At 10-year follow up evaluating symptomatic defects in athletes, patients treated with an OAT procedure had better knee scores, lower number of failures, and a lower rate of osteoarthritis development compared to those treated with microfracture. Furthermore, these patients treated with an OAT procedure had a higher rate of return to sports at their pre-injury level and maintained that level of activity longer than the microfracture group [87]. Disadvantages include the potential for donor-site morbidity and limitations on the size and number of grafts available. Typically, this is an option for lesions less than 2.5 cm2.
Fresh Osteochondral Allografts
Fresh osteochondral allografts have a fairly extensive clinical history, extending over three decades [88–94]. Allograft transplantation is currently gaining in popularity due to increasing appreciation that it reliably restores viable hyaline cartilage with normal architecture when compared to alternative treatment options for larger defects [94, 95]. Although there are logistic issues associated with obtaining allografts, including waiting for an appropriate graft, the procedure itself is not very technically demanding in most cases. The technique can be accomplished with commercially available instrumentation systems versus preparation of a customized “shell” graft (Fig. 23.8). The technical aspects of the procedure have been well described elsewhere and will not be described here [95]. Fresh allografts are most useful in treating larger chondral or osteochondral lesions (> 2.5 cm2), but can also be utilized for smaller defects in some cases. This is especially appealing in a multiligament-injured knee.
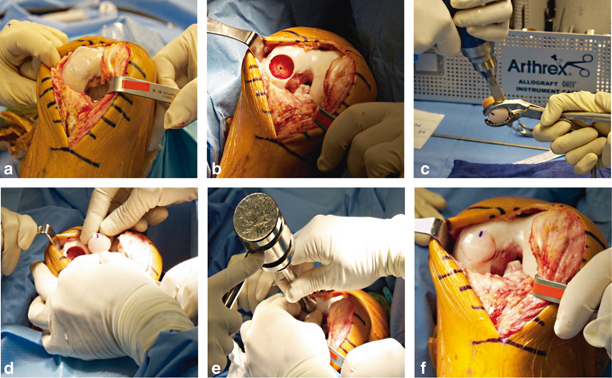

Fig. 23.8
a Large chondral lesion of the medial femoral condyle treated with a microfracture at the initial ligament reconstruction. The patient had persistent symptoms medial despite a stable knee. The lesion was revised to a fresh osteochondral allograft. b Medial femoral condyle following preparation of recipient site. c and d Preparation of fresh osteochondral allograft. e Gentle impaction of the allograft. f Fresh osteochondral allograft seated within the recipient site. (With kind permission from Springer Science + Business Media: The Multiple Ligament Injured Knee [31])
The long-term success of osteochondral allografts is dependent upon preservation of the hyaline cartilage surface, healing of the osseous base to the host bone, and maintenance of structural integrity during the remodeling process [94, 96]. Investigators have shown that chondrocyte viability is paramount in order to maintain the normal extracellular architecture of hyaline cartilage and to prevent the development of degenerative joint disease, but the acceptable degree of chondrocyte viability required is unknown at this time [97, 98]. Although nonviable cartilage will appear grossly normal for a period of time, it will not maintain its histologic, biochemical, or biomechanical properties. As a result, the cartilage will fibrillate, develop clefts, and erode over time [97, 98]. It is important to note that current “fresh” allografts are actually refrigerated for a period of time prior to implantation, in contrast to historical fresh allografts, which were transplanted much closer to time of procurement [99].
Immune compatibility testing and postoperative immunosuppression are not required with osteochondral allograft transplantation despite the fact that chondrocytes and subchondral bone have both been shown to have immunogenic potential [100–103]. Chondrocytes are surrounded by a matrix that isolates them from the host immune cells and makes them relatively “immunologically privileged” [90, 91]. Although donor cells within the osseous component are immunogenic, their immunogenicity is muted and probably not clinically significant in most patients [104, 105]. However, the surgical trauma and the graft itself stimulate a local inflammatory response [106]. This response is primarily directed against the bone constituent of the graft that contains the marrow elements and other immunogenic elements [107]. In general, the osseous component of osteochondral allografts retains its structural integrity and is replaced with host bone via creeping substitution over a period of years [108–111]. If the nonviable bone trabeculae cannot withstand mechanical stresses during the remodeling process, subchondral microfracture, collapse, and fragmentation may occur [94]. Unless there is a need to restore deficient subchondral bone, currently most surgeons have evolved to preparing the graft with less than 6 mm of bone.
Long-term chondrocyte viability and clinical success following osteochondral allograft transplantation has been shown in multiple reports [112–116]. Researchers have biopsied transplants at various time intervals following the index procedure with relatively high rates of chondrocyte viability [113, 116]. This potential for long-term survival supports the use of osteochondral allografts in an attempt to maintain the extracellular matrix and thus prevent long-term articular degeneration within the graft. Although no reports have focused on the PCL or multiligament patient, multiple authors have published on the outcomes of osteochondral allografts in younger patient populations with relatively good success, and a high percentage of patients may be able to return to sport at their pre-injury level, especially in patients < 25 years of age with symptoms lasting less than 1 year [95, 110, 111, 113, 115, 117]. New conflicting data, however, suggest that allografts, similar to other treatment options in this often challenging group, are often not successful in getting patients back to their pre-injury level, especially in a population with physically demanding occupations. In a 2013 study looking at active duty military personnel, 42 % of patients were unable to return to active duty following allograft transplantation, and only 5.3 % of patients were able to return to their pre-injury level [118]. Failures do occur with this technique and may increase with follow-up intervals as with any resurfacing procedure. Failures tend to be more related to the osseous component than the cartilage component and may include fragmentation and collapse [94]. Nonunion has not been a significant clinical problem especially with the dowel graft technique. Failure in this difficult patient population can often be the result of progressive overall joint degeneration and not specifically related to graft failure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








