Articular Cartilage: A Brief Review of its Structure, Function, and Repair
Wayne H. Akeson MD
William Bugbee MD
Few mechanical devices even remotely approach the durability and efficiency of cartilage.
The typical response to cartilage injury in which the subchondral plate is fractured is the formation of fibrocartilage, a scarlike tissue unsuited to the support of compressive loads and shear forces.
The pattern of collagen fibrils within articular cartilage is well suited to the functional requirements of the tissue.
Loss of the densely packed collagen mat at the surface of cartilage in weight-bearing regions is the prelude to fibrillation, accelerated wear, and ensuing degenerative arthritis.
Collagen is the key protein in musculoskeletal stability. It provides the mechanical properties to connective tissue, and it constitutes 65% to 80% of the mass by dry weight of such connective tissues as tendons, ligaments, skin, joint capsules, and cartilage.
Attempts to achieve cartilage repair, as in surgical arthroplasty, do not successfully regenerate cartilage and seldom produce completely satisfactory clinical results. The collagen fiber architecture of the arthroplasty repair tissue is disordered throughout the deep layers and lacks the membranelike characteristics so important to the surface layer of articular cartilage. These are major factors contributing to failure of cartilage regeneration.
Cartilage is a unique tissue that unless injured, provides virtually frictionless mechanical motion throughout the latter decades of life. Few mechanical devices even remotely approach the durability and efficiency of cartilage. The purpose of this chapter is to provide a brief account of the structure, composition, and mechanical properties of articular cartilage. Such an account is basic to understanding both the function of cartilage and the therapeutic goals of cartilage restoration by any of the present (and future) attempts at surgically induced regeneration.
The typical response to cartilage injury in which the subchondral plate is fractured is the formation of fibrocartilage, a scarlike tissue unsuited to the support of compressive loads and shear forces (1,2). If the subchondral plate is not fractured, attempts at healing rely on articular cartilage cells, the response of which to injury is consistently and completely ineffectual (3).
Given the elegant precision of the morphological and compositional interdependence of articular cartilage, it is not surprising that attempts at effective regeneration of articular cartilage have frustrated clinicians and basic scientists alike. Mature mammalian cartilage cells, which constitute only 2% of the total volume of the tissue, have lost the ability to dedifferentiate. This property of cartilage cells is shared with the universe of other mammalian cells resulting from evolutionary progress. This circumstance likely is a controlling factor in the limitation of the biological response of articular cartilage to injury.
As mentioned, articular cartilage is a unique tissue in many respects, but especially with regard to its structural, metabolic, and functional interactions. Articular cartilage possesses unparalleled biomechanical functional efficiency, and this efficiency is derived from design features that are marveled at by physicians and engineers attempting to design artificial substitutes for diseased joints. For example, the articular cartilage lubrication efficiency is an order of magnitude superior to the best bearing surfaces known to modern engineering. Such efficiencies are achieved in spite of stringent limitations imposed on the tissue, such as the
lack of blood supply and a tissue thickness that measures a few millimeters at most. Couple these points with a limited repair capability and the consequent requirement that the tissue survive a lifetime of use and then the question becomes, “How can synovial joints survive as long as they do?” The thrust of this chapter will be to describe the morphological, biochemical, and physiological interactions of the cartilage matrix to provide insight regarding the basis for successful long-term survival of cartilage and the requirements for its successful repair or regeneration.
lack of blood supply and a tissue thickness that measures a few millimeters at most. Couple these points with a limited repair capability and the consequent requirement that the tissue survive a lifetime of use and then the question becomes, “How can synovial joints survive as long as they do?” The thrust of this chapter will be to describe the morphological, biochemical, and physiological interactions of the cartilage matrix to provide insight regarding the basis for successful long-term survival of cartilage and the requirements for its successful repair or regeneration.
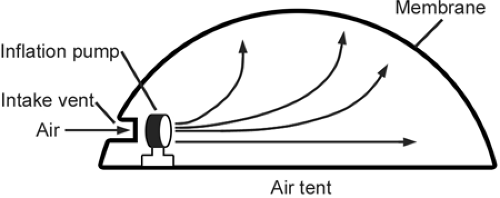
A useful phenomenological concept that is helpful in understanding the function is air tent, which is a structure used as a cover for recreational areas, such as swimming pools and tennis courts, or as a temporary cover for exhibitions (4) (Fig 3-1). The functional requirements for the air tent are (a) an inflation pump or fan, (b) an intake tube for the inflation medium, (c) the inflation medium (air), and (d) the fabric required to contain the pressurization and to provide the cover. The pump must be working constantly to maintain expansion of the system because of inevitable leaks through the fabric. In the case of cartilage, the surface membrane (i.e., the fabric) consists of the fine collagen fibril network concentrated at the articular surface. The inflation pump of cartilage is the proteoglycan molecular structure, and the inflation medium is an ultrafiltrate of synovial fluid. Cartilage, of course, has no single intake vent for the inflation medium to enter. Rather, fluid inflating the tissue enters through a myriad of microscopic pores at the surface; these are the same pores from which the fluid exits when compressed. These elements are interrelated, and a deficiency in any of them will result in failure of the system. In the case of the air tent, a tear in the fabric for which the pump is not able to compensate will result in collapse of the tent. Or, if the pump fails, the tent will gradually collapse as pressurized air leaks through the pores of the fabric.
More specifically, the fabriclike structure at the cartilage surface, consisting of fine collagen fibrils packed tightly in a matted pattern parallel to the surface, is much different from that seen in the deeper layers, where fibers become thicker, their orientation becomes more vertical, and the spaces between the fibers increase. The surface “fabric” of cartilage has tiny pores that permit fluid and small molecules access to and egress from the tissue but that block the movement of large molecules. The inflation medium in articular cartilage is, of course, fluid rather than air. The cartilage fluid is in equilibrium with the synovial fluid, which is essentially an ultrafiltrate of plasma. The fluid in articular cartilage is significantly pressurized. Calculations by Ogston (5) led him to conclude that articular cartilage is inflated to the equivalent of “motor tire pressure.” The pump for this pressurized system is not intuitively obvious, but its presence has been established without doubt by modem techniques of rheology and biophysics. The pump for the articular cartilage system is chiefly aggrecan, a proteoglycan molecule that becomes linked to hyaluronan to form a huge molecule designated as a proteoglycan aggregate. This huge macromolecule is locked within the articular cartilage fibrillar matrix by its large size and volume.
In its state of equilibrium, the expansion pressure in the articular cartilage system is in balance with the resisting tension of the collagen fibers; however, the balance can be upset by an externally applied load. If the external pressure exceeds the internal pressure, fluid will flow outward until a new equilibrium is reached. As the proteoglycan molecules become compressed, their charges become more concentrated, and this causes the fluid pressure within the cartilage to be increased until a new equilibrium is reached. The theoretical analysis of the fluid flow patterns and viscoelastic properties under various loading conditions has been studied extensively. This fluid movement is of great interest, because it explains the mechanism of several fundamental properties of the articular cartilage system, including lubrication, load bearing, and nutrition.
As indicated above, the proper evaluation of attempts at repair requires fundamental knowledge of articular cartilage form, composition, and biomechanical characteristics. The collagen matrix of normal articular cartilage, its proteoglycan and proteoglycan aggregate, and the movement of fluid within cartilage are described in greater detail in the following sections with respect to its morphologic, biochemical, and functional features.
Morphology of the Collagen Framework
The pattern of collagen fibrils within articular cartilage is well suited to the functional requirements of the tissue. The air-tent analogy described earlier requires that a pressurized internal medium be constrained from expansion by a
membrane. A matted surface layer of collagen fibrils provides this membranelike function.
membrane. A matted surface layer of collagen fibrils provides this membranelike function.
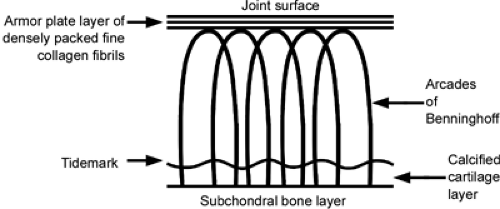
The collagen pattern in the deeper layers of the cartilage surface is morphologically quite different from the surface pattern. In 1925, Benninghoff (6) described an arcade pattern of organization for articular cartilage collagen (Fig 3-2). This pattern has subsequently been challenged with respect to the precise accuracy of the proposed scheme (7,8,9). The concept is at least partially correct, however, and it is useful in understanding the function of cartilage. The surface fibrillar pattern clearly differs from that of fibers in deeper layers (10). The surface collagen fibrils are smaller (diameter, 30–32 nm) and more closely packed than in the middle and deeper layers. The surface pattern of the collagen framework as described has been recognized implicitly for decades by the term “armor plate” layer, referring to the tough, resilient, skinlike cartilage surface. The collagen concentration is greatest at the surface, where the small fibrils are compacted tangentially. This arrangement creates a small pore size, which has been calculated by McCutchen (11) to be approximately 6 nm. The largest molecule that can traverse a pore of this dimension is hemoglobin. Small ions and glucose, for example, easily traverse these pores, but larger molecules, such as most proteins and hyaluronan (hyaluronic acid), do not enter cartilage in significant amounts under normal conditions.
Collagen fibers in the intermediate layers are no longer oriented tangentially to the surface but, rather, are directed obliquely or randomly. They are larger than the surface fibrils, with most ranging between 40 and 100 nm. The deepest fibrils are the largest in cartilage. They are disposed perpendicularly relative to the joint surface, and they perforate the calcified basal layers of cartilage through the tidemark regions and, eventually, enter the subchondral bone layer, where they are firmly attached, much as in the attachment of the Sharpey fibers of ligament to cortical bone. This feature is crucial for cartilage to be able to resist shearing forces, which otherwise would tend to peel the cartilage away from the subchondral surface.
It has been well demonstrated clinically that loss of the densely packed collagen mat at the surface of cartilage in weight-bearing regions is the prelude to fibrillation, accelerated wear, and degenerative arthritis. This seems completely logical, because the coarse, widely spaced fibrils in deeper layers that are principally oriented vertically are poorly suited for constraining the swelling forces that are generated by the matrix proteoglycans. The term fibrillation describes the tendency of these fibrils to be split vertically all the way to their subchondral attachment, much as wood splits along the grain of its fibers. The villuslike strands so exposed collectively resemble a shag rug, and the individual strands are prone to tear off at the base when mechanically loaded and exposed to shear stresses. Clearly, the description of an “armor plate” applies well to the normal surface mat of collagen fibrils, and loss of this layer no longer permits the cartilage to function as a pressurized unit suited to weight-bearing.
Evidence supporting the fibril pattern of collagen orientation derives from several types of observation, including routine histology, transmission-electron microscopy, scanning-electron microscopy, and the demonstration of Hultkrantz lines (12). Hultkrantz lines typically are observed on the surface of cartilage and are analogous to the Langer lines of skin (13). These lines become visible when the surface of cartilage is pricked with a pin. Coating the cartilage surface with India ink and then wiping it dry best demonstrates the puncture defects. Hultkrantz noted many years ago that the puncture holes appeared as slits rather than round holes. Furthermore,
these slits have axes that generally are perpendicular to the principal axis of movement of the joint. Hultkrantz lines therefore are different for each joint of the body. Mechanical tensile tests have confirmed that the Hultkrantz lines indicate the preferred orientation of the collagen fibrils at the surface of the joint resists tensile forces. Bullough and Goodfellow (14) have shown this characteristic of joint surfaces in polarized-light experiments illustrated in Figure 3-3. For interested readers, the pattern of matrix and cellular organization of articular cartilage has been described in greater detail by Wong and Hunziker (10).
these slits have axes that generally are perpendicular to the principal axis of movement of the joint. Hultkrantz lines therefore are different for each joint of the body. Mechanical tensile tests have confirmed that the Hultkrantz lines indicate the preferred orientation of the collagen fibrils at the surface of the joint resists tensile forces. Bullough and Goodfellow (14) have shown this characteristic of joint surfaces in polarized-light experiments illustrated in Figure 3-3. For interested readers, the pattern of matrix and cellular organization of articular cartilage has been described in greater detail by Wong and Hunziker (10).
Collagen Chemistry
The molecular structure of collagen has been of considerable interest for more than a century, because it is the principal structural protein (by mass) for all mammals. It constitutes 65% to 80% of the mass by dry weight of such specialized connective tissues as tendons, ligaments, skin, joint capsules, and cartilage. It is the only protein with significant tensile force–resisting properties with the exception of elastin, the functional role of which is quite different. Therefore, collagen is the key protein in musculoskeletal stability, providing the mechanical properties that impart the “connect” to connective tissue.
The tensile force–resisting properties of cartilage derive from the precise molecular configuration of the collagen macromolecule. This molecule is one of the largest in the body, forming a rodlike structure 300 nm in length and 1.5 nm in diameter. These rods are termed tropocollagen (15). They are assembled in a three-dimensional array in the extracellular environment, being influenced, somehow, by environmental stresses and additional biological factors of which the full details regarding their nature are still unclear. The sum of the extracellular influences somehow affects the orientation and size of fibrils that are assembled from the tropocollagen units (15,16,17,18,19). The tropocollagen assembly typically is patterned in a quarter stagger.
The α chains are not identical among species or within a single species. Early data regarding mammalian skin collagen demonstrated two types of α chains, α1 and α2, which were present in a ratio of 2:1 (15). Miller and Matukas (20) were the first to show that cartilage possesses a collagen that is different in composition from that in most fibrous connective tissues. This collagen contains a different type of α1 chain, which they termed α1, type II. The collagen in most cartilages consists of three such identical chains, and the abbreviated nomenclature is now αl [II]3, or type II collagen (21).
At least 27 different types of collagen products of 40 genes have been described in vertebrates (21). These collagens can be divided into two major classes on the basis of their primary structure and supramolecular assembly: the fibril-forming collagens, and the non–fibril-forming collagens. The fibril-forming collagens include types I, II, III, V, and XI (22). Each of these types has a long, central, triple-helix domain without any interruptions in the glycine-x-y sequence, where x and y are amino acids. The rest of the collagens belong to the non–fibril-forming class. Although they vary in size, they share the feature of having imperfections in the glycine-x-y sequence. Within this class, type IX, XII, and XIV collagens form a subgroup called the fibril-associated collagens with interrupted triple helices (FACIT). They are associated with type I or II collagen fibrils, and they play a role in the interaction of these fibrils with other matrix components. Although their sizes and primary structures vary, they share several common structural features. Type XVI collagen appears to be a member of this group (23). Summaries of the makeup and distribution of the collagen types accepted at present have been provided in several recent review articles (22,23,24,25).
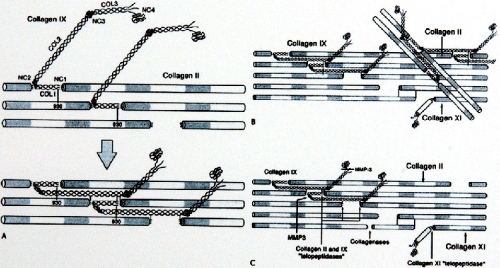
The significance of the type II collagen to cartilage is not yet known. It is a heteropolymer with type IX collagen molecules covalently linked at the surface and type XI collagen molecules forming a filamentous template at the core. The principal differences between this collagen and the more common type I collagen that is found in fibrous connective tissue involve the number of hydroxylysine molecules and the presence of a small number of residues of cysteine. The type II collagen fibrils are thinner near the articular surface and the tangential zone than that are in the deeper zones, and the collagen concentration is greater at the surface. Evidence is accumulating that type IX and XI collagens make critical contributions to the organization and mechanical stability of the type II collagen fibrillar network (Fig 3-4).
Type IX collagen makes up approximately 10% of the collagen protein in fetal mammalian articular cartilage, but the amount decreases to approximately 1% in adult tissue. The molecule also is categorized as a proteoglycan, because it was originally demonstrated in chicks that single site for attachment of chondroitin sulfate exists on the type IX collagen molecule. Type IX collagen also is characterized by the presence of four globular domains in the triple-helix structure (26). In bovine articular cartilage, type IX collagen is found on the surface of type II collagen and appears to be linked covalently to at least one molecule of the type II collagen triple helix (26). From this evidence, type IX collagen is believed to provide a covalent interface between the surface of the type II collagen fibril and the interfibrillar proteoglycan domain. Another theory is that type IX collagen provides interfibrillar linkages between type II collagen fibrils and, therefore, may enhance the mechanical stability of the fibrillar network.
Type XI collagen makes up approximately 3% of mature articular cartilage collagen. It has a single globular domain on one end of the triple helix, and it is located within the type II collagen fibrils (21). Diagrams illustrating the Type II/IX/XI collagen heteromer is shown in Figure 3-4. Other fibrous collagens found in articular cartilage are type VI and X collagens. Both have a short helix. Type X collagen is found only in hypertrophic zones of growth plates. Type VI collagen has a globular domain on each end (21). Type VI collagen is unique in that it has no aldehyde cross-link and has arginine–glycine–aspartic acid (RGD) sequences in each α chain; these sequences are important in cell attachment (27). It binds to hyaluronan (28) and to fibronectin (29), and it has been identified in the perilacunar matrix surrounding chondrocytes.
The fundamental process of collagen formation the chondroblasts and chondrocytes is nearly identical to its synthesis by the fibroblast and fibrocyte. The collagen turnover in cartilage proceeds at a rate not unlike that seen in connective tissue of the fibrous type. Because significant collagen synthesis occurs in adult cartilage, the control processes for spatial orientation of the product, although poorly understood, clearly are of crucial importance.
It is a source of frustration to surgeons and their patients that attempts to achieve cartilage repair, as in surgical arthroplasty, do not successfully regenerate cartilage and
seldom produce completely satisfactory clinical results. The collagen fiber architecture of the arthroplasty repair tissue is disordered throughout the deep layers and lacks the membranelike characteristics that are so important to the surface layer of articular cartilage. These are major factors contributing to the failure of cartilage regeneration. Details regarding the biology of the cartilage repair process are described later in this chapter.
seldom produce completely satisfactory clinical results. The collagen fiber architecture of the arthroplasty repair tissue is disordered throughout the deep layers and lacks the membranelike characteristics that are so important to the surface layer of articular cartilage. These are major factors contributing to the failure of cartilage regeneration. Details regarding the biology of the cartilage repair process are described later in this chapter.
Collagen Cross-Links
Stabilization of collagen occurs extracellularly, after its assembly into the quarter-stagger arrays that make up filaments, fibrils, and fibers. The stabilization and ultimate tensile strength of the fiber structure are thought to result mainly from the development of intramolecular and intermolecular cross-links. The former occur between α chains of the individual tropocollagen molecule and the latter between adjacent tropocollagen molecules. The cross-links result from enzyme-mediated reactions involving mainly lysine and hydroxylysine. The details of the bifunctional, trifunctional, or quadrifunctional cross-links so created are beyond the scope of this discussion but are available in several relevant reviews (30,31,32,33,34,35,36,37,38,39).
Sulfated proteoglycan macromolecules constitute 12% of articular cartilage dry weight. The proteoglycans of articular cartilage serve as the “pump” of the highly pressurized cartilage system. As mentioned earlier, the characteristics of the proteoglycan molecules that permit this crucial function include their very large size and resulting immobility within the collagen fibril meshwork; their densely concentrated, fixed, negative sulfate and carboxyl charges; and the large number of hydroxyl groups contained. These characteristics collectively serve to attract water and small positively charged ions into the cartilage. Donnan osmotic pressure results from this process. The negative charges on the proteoglycan molecules naturally create repulsive forces between each other, which are termed chemical expansive stresses. The sum of the Donnan osmotic pressure and the chemical expansive stress constitutes the cartilage swelling pressure (17). Ogston (5) noted the rough equivalence of the pressure within articular cartilage with “motor tire pressure”!
The extraordinary size of the proteoglycan aggregate molecules of articular cartilage is achieved by supra-assembly of three different types of linear-chain molecular species: sulfated glycosaminoglycans, a core protein, and hyaluronan (a nonsulfated glycosaminoglycan). The purpose of this section is to describe briefly the chemical structure of the functionally vital proteoglycan and its proteoglycan aggregate and to illustrate the manner in which the functional role derives from the chemical structure.
Glycosaminoglycans
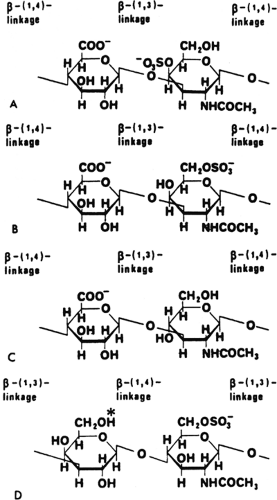
Figure 3-5 shows the repeating disaccharide unit for the glycosaminoglycans of articular cartilage: chondroitin-4- sulfate, chondroitin-6-sulfate, hyaluronan, and keratan sulfate. In most of the glycosaminoglycan molecules, hexosamine alternates with another sugar polymerized in a repeating disaccharide pattern. The predominance of the amine group
in this configuration is the reason for the use of “amino-” in the term glycosaminoglycan. The common features of the group are obvious at first glance. In particular, the location of the N-acetylamine group at the C2 of the hexosamine is common to all the disaccharides shown. The hexosamine is galactosamine in three of the four cases; keratan sulfate possesses glucosamine as the alternating hexosamine. All except hyaluronan are sulfated at the C4 or C6 position of the hexosamine. Each disaccharide contains at least three hydroxyl groups. All except keratan sulfate contain uronic acid as the second element of the disaccharide, with a carboxyl terminal at C6. Keratan sulfate possesses galactose rather than uronic acid as the second half of the disaccharide (17).
in this configuration is the reason for the use of “amino-” in the term glycosaminoglycan. The common features of the group are obvious at first glance. In particular, the location of the N-acetylamine group at the C2 of the hexosamine is common to all the disaccharides shown. The hexosamine is galactosamine in three of the four cases; keratan sulfate possesses glucosamine as the alternating hexosamine. All except hyaluronan are sulfated at the C4 or C6 position of the hexosamine. Each disaccharide contains at least three hydroxyl groups. All except keratan sulfate contain uronic acid as the second element of the disaccharide, with a carboxyl terminal at C6. Keratan sulfate possesses galactose rather than uronic acid as the second half of the disaccharide (17).
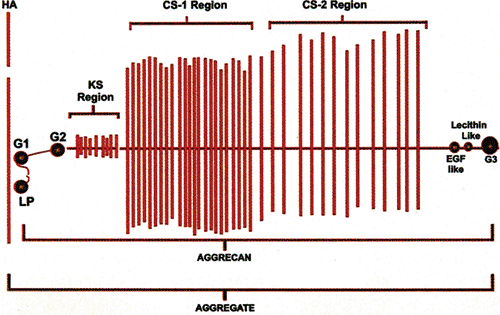 Fig 3-6. Aggrecan molecule. This diagram shows the assembly of chondroitin sulfate (CS-1 and CS-2) and keratan sulfate molecules onto a protein core linear structure, which in turn becomes fixed to hyaluronan via specialized G-1 and LP regions. The various specialized linkage regions are composed of highly specific molecular configurations (see text). The attachment of the core protein and its polysaccharide side chains (aggrecan) to hyaluronan to create the aggregate structure is shown. Typically, a high concentration of keratan sulfate, with its smaller number of disaccharide units near the attachment site of the core protein to hyaluronan, is present. (The CS-1 and CS-2 regions are not drawn to scale; usually, they are twice as long as shown.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|