provide stability in all three planes. When considering joint replacement, there are certain anatomic stabilizing features that need to be considered. First, the curvature of the talar dome in the ankle mortise has stabilizing qualities in anterior to posterior shear loads. Additionally, frontal plane stability is a result of congruent medial and lateral talomalleolar articulations. In the clinical setting, any condition that alters the ankle geometry (i.e., surface incongruity) or through extra-articular malalignments (i.e., end-stage hindfoot pronation) may affect ankle stability under weight-bearing conditions (19).
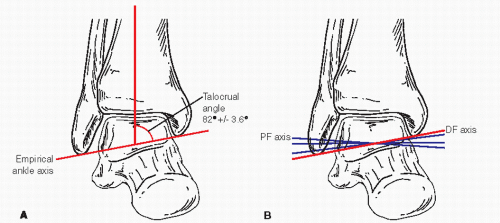 Figure 52.1 A: Diagram of ankle with empirical axis through the talocrural line. B: Diagram showing actual axes of plantar and dorsal flexion. |
take a more lateral course deep to peroneus tertius muscle and pass anterior to lateral malleolus. In the second variation (˜1% occurrence), the perforating peroneal assumes the position of the AT artery. In the third variation (1% occurrence), the AT artery will give off a lateral branch to take over function of perforating peroneal. While the AT artery shows mild variation, the dorsalis pedis is consistent in its pattern.
The preoperative determination of ultimate outcome for the individual patient is an imperfect science to date since there are many contributing factors that are known and unknown that collectively determine clinical outcome. Yet as the collective experience with implant arthroplasty increases, more definitive and predictive parameters will emerge.
from loss of joint space and contour. Motion demands are shifted to adjacent and distal joints of the foot. With the loss of ankle motion, these joints are subjected to abnormal forces including torque and excursions, which are at variance to their natural axes of motion. In a recent prospective study, there is a 10.8% increase in combined subtalar and medial column motion within 33 months after isolated ankle arthrodesis (73). In long-term follow-up, ipsilateral adjacent joint arthrosis has been documented to consume Lisfranc, midtarsal, and subtalar joints and not the ipsilateral knee (74,75 and 76). Functional
impairment of end-stage ankle arthritis that is severe has been measured using a Musculoskeletal Functional Assessment (MFA) outcomes measurement tool (77). This study evaluated 426 patients with ankle arthritis and compared them to a general population group, and an ankle/foot trauma group (9 to 12 months after surgery). The results showed MFA scores that were more than triple the scores in the general population, and dysfunction was more than double than patients having a recent history of trauma.
| |||||||||||||||
Which is the better implant design for this patient type: a low-profile device that is placed in denser periarticular bone or a larger device that can maximize surface area for better load dispersion?
Is a low-profile two-component device going to be more susceptible to premature aseptic loosening or subsidence than a similar-sized mobile bearing device due to increased loads placed at the bone-implant interface?
paucity of high-quality evidence that relates specifically to the effects of smoking on the success of total joint replacements. Conflicting reports relate that smoking can cause implant loosening and affect device survivorship (102,103). Further research is needed to isolate and confirm the true effects of smoking on total joint replacement. There remains concern with smokers if any type of arthrodesis is part of the surgical plan. Furthermore, it is well known that smokers have more difficulties with wound healing as compared with nonsmokers and would likely carry more risk with delayed incisional healing in TAR.
TABLE 52.2 Second-Generation Total Ankle Replacement Clinical Results | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree