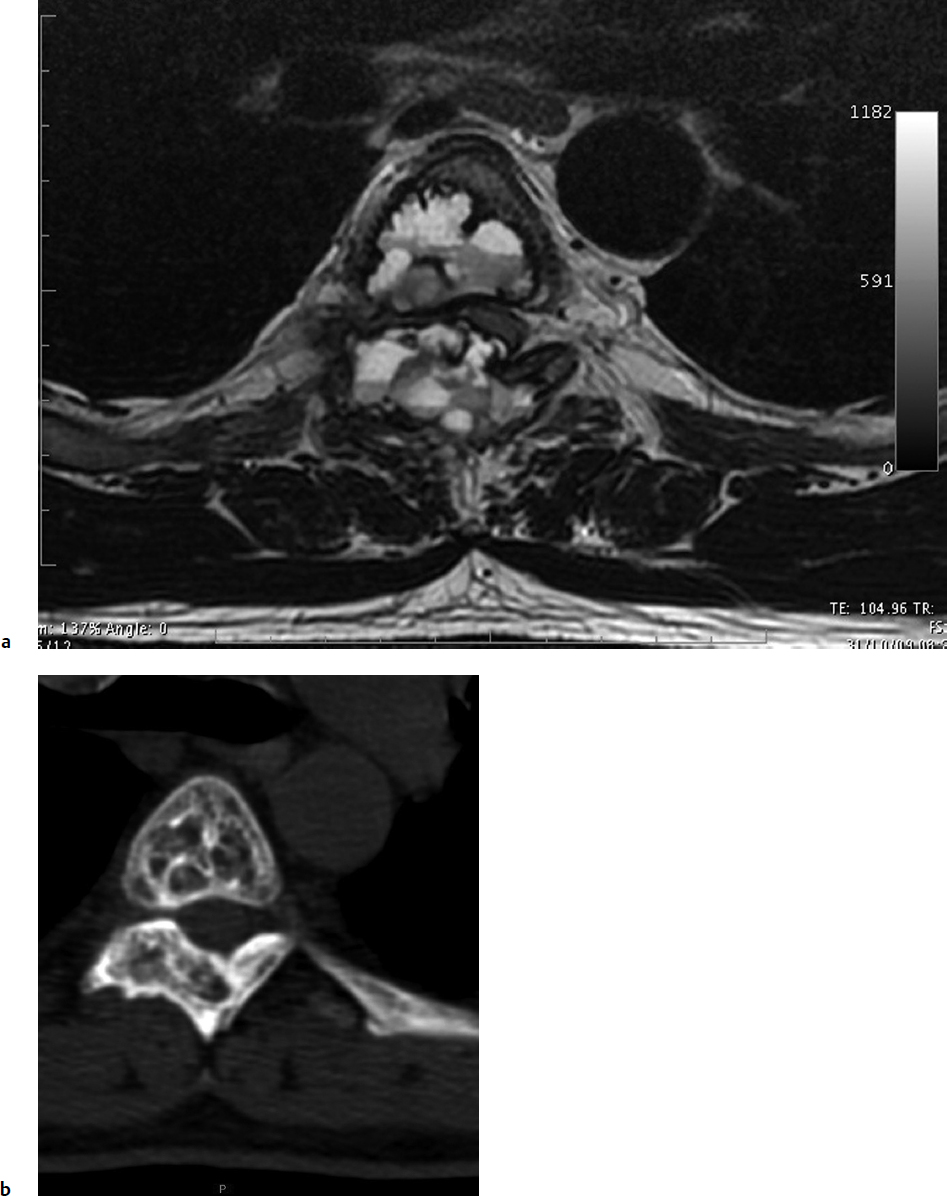
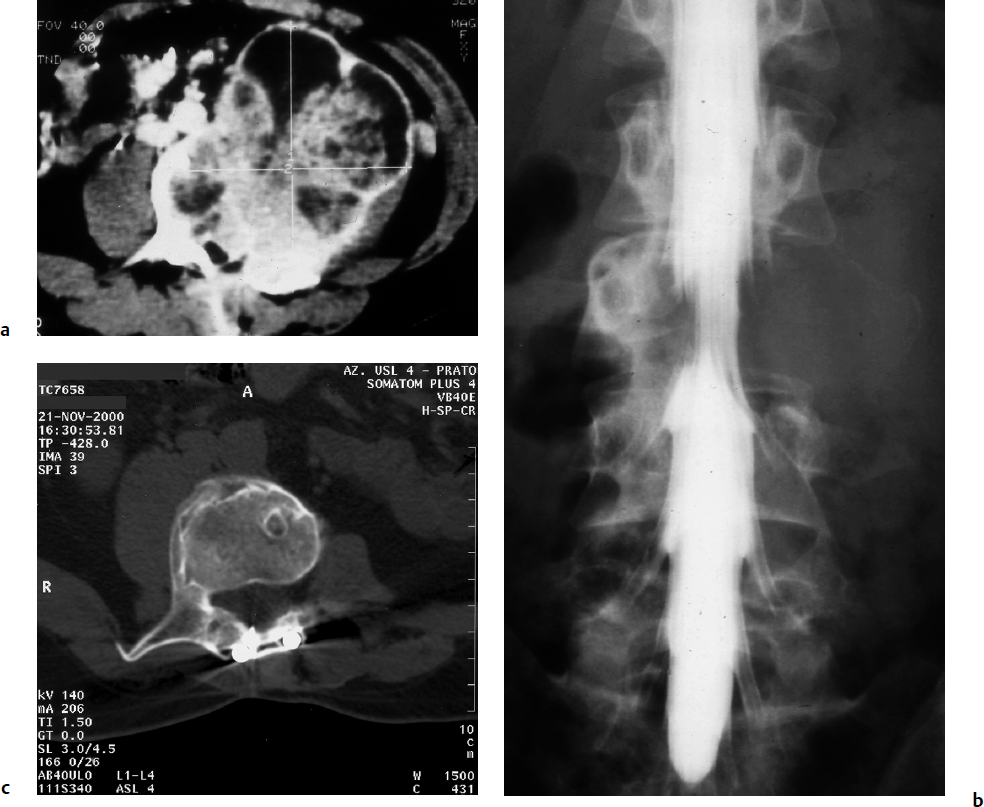
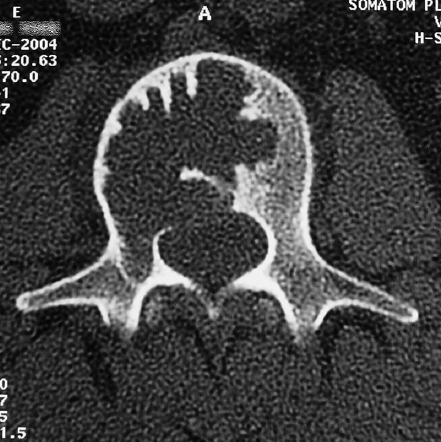
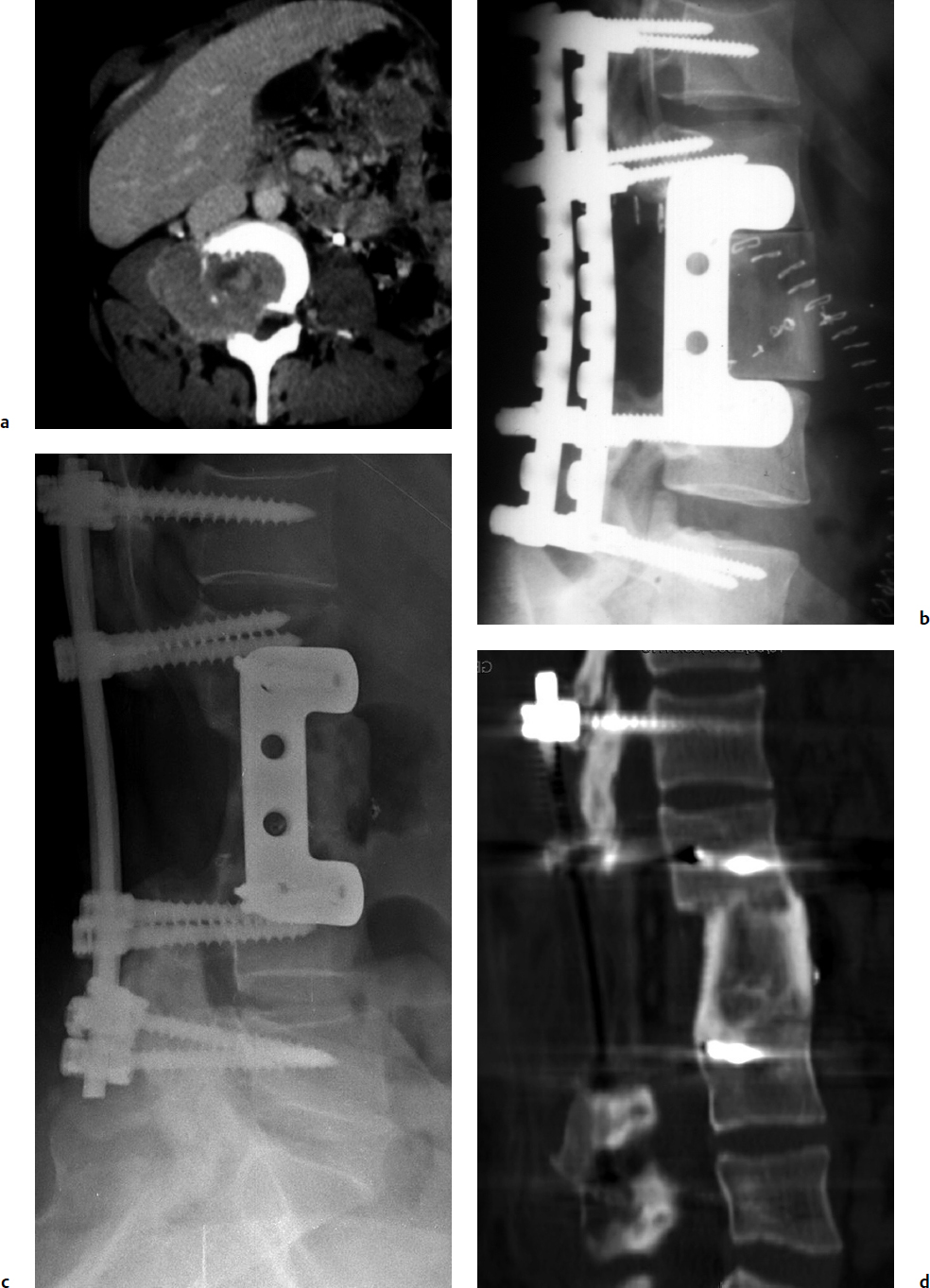
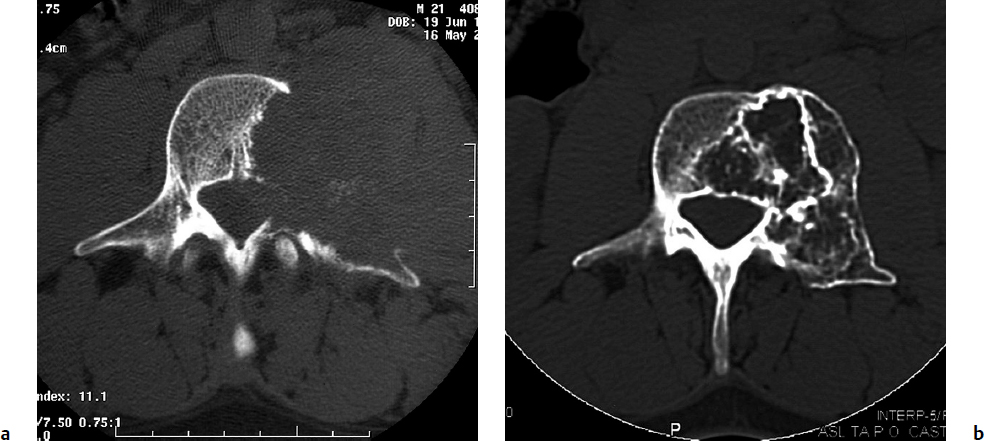
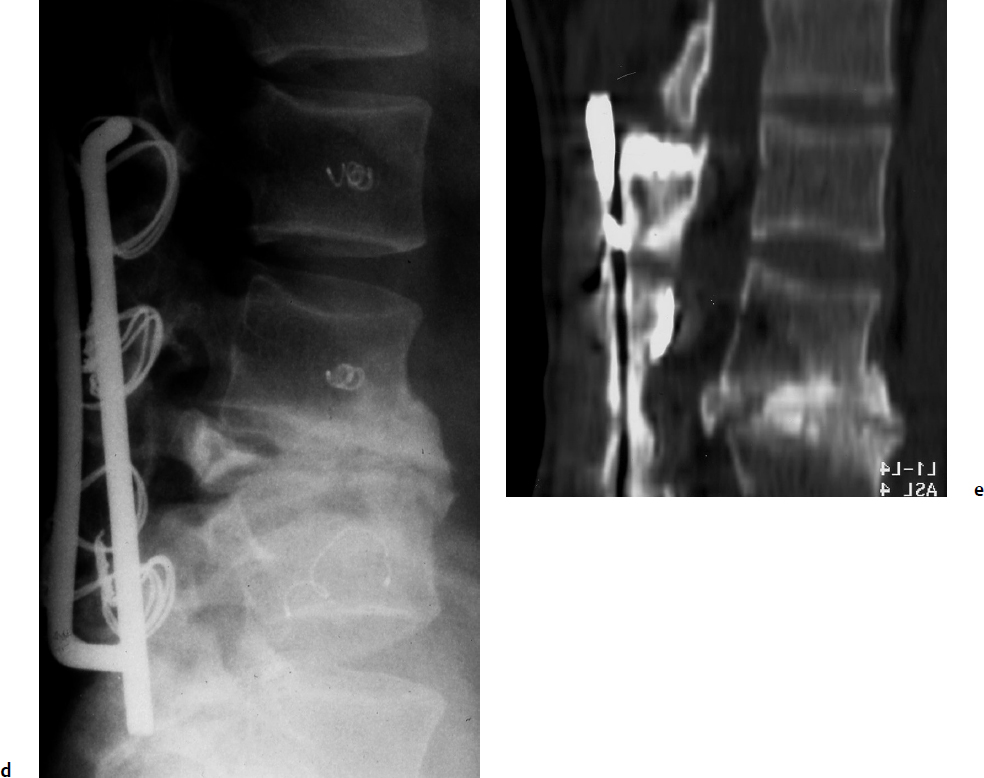
7 Many bone tumors include giant cells in their cytoarchitecture. Among them, aneurysmal bone cysts (ABCs) and giant cell tumors (GCTs) are the most frequently observed in the spine. Sometimes these tumors are difficult to differentiate from each other due to similar clinical findings, imaging findings, and debatable histological pattern. Therefore, this justifies the inclusion of these tumors in the same chapter. Aneurysmal bone cysts were first described by Jaffe and Lichtenstein1 in 1942, when it was differentiated from hemangiomas and other tumors containing giant cells. According to the World Health Organization (WHO), ABC is defined as a destructive, expansile, benign neoplasm of bone, composed of multiloculated blood-filled cystic spaces separated by connective tissue septa containing osteoclast-type giant cells, fibroblasts, and reactive woven bone.2 The ABCs represent about 1.0 to 1.4% of all primary bone tumors. The meta-epiphyseal regions of long bones are most likely to be affected, especially the femur and tibia.1 Most cases of ABC are identified in the first two decades of life, and predominantly between 10 and 20 years of age. It rarely occurs after 30 years of age, even though it has been described in patients older than 50 years old. Approximately 10 to 30% of ABCs involve the mobile spine, most commonly in the thoracic and lumbar spine. The most common symptom is local pain. Swelling is frequent when an ABC arises from superficial bones, but this rarely occurs in the spine. ABCs mostly arise from the posterior elements, but frequently invade the pedicles, the epidural space, and the vertebral body, often resulting in pathological fractures and neurologic compromise. Radiographs typically show an expansile osteolytic cavity with strands of bone forming a bubbly appearance. The frequently observed “level” images are caused by the double density of the cyst’s contents (blood and membranes) and may be considered pathognomonic of ABC. The cortex is often eggshell thin and blown out (Fig. 7.1). The lesion is often extremely vascular, but this feature is not often angiographically appreciated even though it is grossly apparent, mainly due to the large number of very thin feeding arteries. Spinal cord or nerve root compression associated with neurologic deficits can be the prominent initial symptom, as some lesions can present with very rapid growth and local aggressiveness. Secondary ABC-like changes are seen in 10% of GCTs (Fig. 7.2), and less frequently in osteoblastomas, chondroblastomas, and fibrous dysplasias that have undergone hemorrhagic cystic changes. These hemorrhagic areas can be observed also in primary malignant tumors (osteosarcoma, angiosarcoma) and even in metastases. Treatment and prognosis in these cases are similar to those of a solid tumor, and accuracy of histological diagnosis is mandatory for appropriate management. The ABCs sometimes have a self-limiting course, evolving to spontaneous healing characterized by disappearance of the double content and ending in ossification of the lytic areas. Turbulent courses, on the other hand, can be observed with huge masses and massive bone erosion. Pathological fractures or spinal cord or nerve compression can follow, sometimes requiring emergency surgery. It is commonly accepted that intralesional excision (curettage) is the best treatment for primary ABCs. This procedure is frequently risky and sometimes complicated by significant interoperative hemorrhage, which can be controlled by full removal of the cyst-lining wall at the time of surgery. In the spine, full surgical removal of the content of an ABC can be challenging and time-consuming, and may expose the patient to profuse bleeding. Many studies have attempted to reduce surgical morbidity and at the same time achieve full local control, with results indicating that preoperative selective embolization (with polyvinyl alcohol or absorbable gelatin or cyanoacrylate) can significantly reduce bleeding and become itself a treatment option.3,4 Fig. 7.2a–e A 26-year-old man presented in 1985 with a giant cell tumor (GCT) and a huge aneurysmal bone cyst (ABC). (a) The huge ABC-like mass is expanding into the abdomen. (b) A cauda equina is compressed by the epidural tumor growth, which was Enneking stage 3. An SAE was performed, which succeeded in reducing the mass, enabling a double-approach gross total excision, followed by conventional radiation therapy. (c) A CT scan 15 years later demonstrated no local recurrence. (d) A standard radiogram 15 years later. (e) A CT scan sagittal reconstruction demonstrated loss of lordosis and fusion. The radiographic result is poor sagittal alignment, but the patient does not complain about pain and participates in sports activities, such as running. At a 25-year follow-up phone contact, the patient reported light back pain, full activity, and no tumor recurrence. Recurrence rates for ABC in the spine have been reported in the range of 10 to 25%. ABC is considered radiosensitive,5 and radiotherapy can be used as single treatment or adjuvant therapy, but radiation can also produce adverse effects on growth in children with consequent late deformities. Additionally, it may have delayed effects on the spinal cord (radiation-induced myelopathy) and increase the risk for sarcomas. In some studies, more aggressive surgery such as en-bloc resection has been advocated as a possible option,6 considering that an extra-lesional surgery is associated with less blood loss and with the highest local control rate. The required excision is frequently so destructive that instrumented fusion must be considered in the operative planning. According to the WHO classification, GCT is a benign but locally aggressive primary tumor, composed of proliferation of mononuclear cells among which numerous macrophages and large osteoclast-like giant cells are scattered.2 GCTs comprise 4 to 8% of all primary bone tumors, and it most commonly occurs between the second and fourth decade of life, with a slight female predominance. Giant cell tumor is most commonly found in the juxta-articular metaphysis of long bones7 and occurs rarely in the spine; the incidence in the mobile spine ranges from 1.4 to 9.4%. If the sacrum is included as part of the spine the incidence rises to 10%. GCT of the spine usually arises from the vertebral bodies, but multi-centric simultaneous presentation has been documented. Imaging patterns include a moth-eaten or irregular radiolucent erosive lesion (Fig. 7.3) with irregular thin sclerotic reaction, sometimes expanding the vertebral contour. Tumor expansion in the soft tissue or in the canal can cause destruction of the peripheral cortex (Fig. 7.4). Secondary ABC-like changes are seen in 10% of GCTs (Fig. 7.2). Pain, mostly occurring during the night, is the most frequent symptom. Neurologic symptoms due to foramen or spinal canal encroachment by the tumor mass are less frequently reported, as are pathological fractures. However, the erosive activity of undetected GCT may ultimately lead to pathological fractures. Ever since GCTs were identified, predicting their behavior has been challenging.7 Various attempts to develop staging systems for the tumor have been unsuccessful.8 According to the Enneking staging system,9 GCT can be found as stage 2 active or stage 3 aggressive benign lesions. Active lesions are characterized by fully erosive patterns typically occurring in the vertebral body, with well-defined borders. Stage 3 aggressive lesions have indistinct margins eroding the cortex, expanding in the surrounding soft tissue, early invading the epidural space. Prolonged disease-free survivals have been reported after curettage and radiotherapy, although some patients may require two or more additional procedures owing to local recurrence. On the other hand, en-bloc excision, when feasible, is curative. Interestingly, GCTs can produce pulmonary metastases even without histological evidence of malignancy. These metastases can be lethal in 25% of cases, but occasionally may regress spontaneously or be controlled by marginal excision. In 20 patients with lung metastases out of 671 patients with GCT observed at the Mayo Clinic, two patients died of the disease. Fig. 7.3 A CT scan demonstrating a fully lytic change with thin osteosclerotic rim at the periphery in a 36-year-old man. The margins are well defined. No soft tissue mass is expanding outside the margin, and there is no epidural extension and no pathologic bone formation. This imaging is consistent with Enneking stage 2. Selective arterial embolization (SAE) can decrease intraoperative bleeding and is therefore mandatory before intralesional excision. Radiation therapy must be considered only as an adjuvant after complete intralesional excision, but high-grade sarcomas are estimated to occur in 5 to 15% of cases treated by this modality. Spinal GCTs have a considerably poorer prognosis than those in the appendicular skeleton, with recurrence rates of up to 80% after treatment. However, this high rate is possibly more related to the technically demanding surgery in the spine than to some intrinsic feature of GCT of the spine. En-bloc resection is associated with the lowest risk of local recurrence, but this technique entails considerable morbidity and a not negligible mortality. Considering that the only successful treatment seems to be en-bloc resection (which is not always feasible and is burdened by high morbidity and mortality), the possible role of local and systemic adjuvant therapies should be investigated. Currently, denosumab (a monoclonal antibody) is particularly promising. Some patterns of ABC imaging are pathognomonic. Multiple bubble structures, each including double content (blood and membranes, whose interface changes orientation according to the gravity), with a variable amount of solid radiolucent tissue is frequently observed. Stage 2 GCTs (so-called active according to the Enneking staging system) have clearly defined margins, sometimes with an ossified border. Stage 3 GCTs (also called “aggressive benign”) have typically fuzzy borders, early epidural invasion, and radiolucent erosion of the vertebra. Even if imaging can be pathognomonic, histological diagnosis should always be performed due to the possibility that ABC is occurring as a secondary lesion in GCT or even in a malignant tumor. Diagnosis should always be achieved by histological and immunohistochemical studies. Biopsy should be performed by trocar under computed tomography (CT) scan control. This technique not only reduces morbidity and tumor contamination of surrounding soft tissues, but also enables taking the most representative sample for the pathologist, as the CT scan can identify the area of solid tissue where enough material for differential diagnosis will be found. Considering that both spontaneous healing10 and progression to malignancy11 are reported in the literature, as well as secondary tumor, such as leukemia, occurring after treatment with radiation therapy, the decision-making process for selecting the most appropriate treatment should include a very careful evaluation of the risk–benefit ratio of each possible treatment. Options for treating ABCs have included simple intralesional excision with or without fixation and bone grafting, gross total excision, en-bloc resection,6 embolization,4 intracystic injections,12 radiation therapy,5 or a combination of these methods. There is no evidence of a better outcome from any of the proposed treatments, but several complications were associated with more aggressive modalities of treatment, including massive bleeding, limitation of growth and range of motion in cases of arthrodesis, secondary malignancies after radiation, and even fatal events. The treatment associated with the lowest recurrence rate is en-bloc resection,6 but this treatment possibly exposes the patient to high surgical morbidity and theoretically must be considered as an overtreatment, considering the benign histological pattern. En-bloc resection should be performed only in very select cases, for example in posteriorly located lesions that are less technically demanding and that expose the patient to less blood loss compared with intralesional excision. Radiation therapy has also been used with good results, but due to the risk of radiation-induced sarcoma and cord myelopathy, this procedure should be considered only when all other treatments fail.4,5 In the literature, SAE was first used to reduce the intraoperative bleeding, and was palliative or curative in very select cases.13 The first case fully treated with SAE was reported in 1990 by DeRosa et al.3 Later, there were several reports of good results in patients treated only with SAE. In a recently published series of seven cases prospectively submitted to sequential embolizations,4 no case required surgery and all evolved to full healing (Fig. 7.5). The technique included microcatheterization and infusion of embolic agents only into the feeding arteries via the microcatheter, thus sparing normal, uninvolved arteries. Most of these cases, however, required multiple sessions—sometimes as many as seven embolizations every 2 months. The longer time required to perform SAE and the exposure to radiation due to repeated angiographies and CT scanning must be considered in the decision-making process. One of the limitations of SAE is the risk of embolization of the artery of Adamkiewicz. In case a feeding artery is detected as a branch of the artery to be embolized, the procedure must be aborted and converted to surgery. An exhaustive literature review was performed in 2009 on 482 articles on ABC.14 Only 94 articles pertained to the spine or vertebrae, mostly consisting of case reports and small case series. Based on such “very low quality” literature, therefore, a weak recommendation suggested selective arterial embolization as a stand-alone modality, whereas a strong recommendation could be given for selective arterial embolization as a preoperative adjuvant option, as it facilitates resection by reducing intraoperative blood loss. In contrast, radiation and chemotherapy were weakly recommended as additional therapy after incomplete resection and recurrence.
Aneurysmal Bone Cyst and Giant Cell Tumor

 Introduction
Introduction
 Aneurysmal Bone Cyst
Aneurysmal Bone Cyst
 Giant Cell Tumor
Giant Cell Tumor
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree