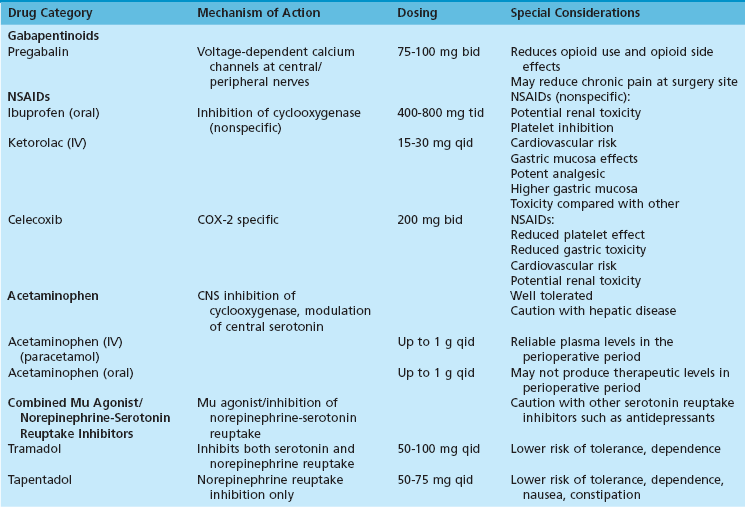
Chapter 5 COMPONENTS OF MULTIMODAL ANALGESIA Nonsteroidal Antiinflammatory Drugs Combining Paracetamol (Acetaminophen) and Nonsteroidal Antiinflammatory Drugs PATIENTS WHO REQUIRE SPECIAL CONSIDERATION Ultrasound-Guided Peripheral Nerve Blocks Continuous Peripheral Nerve Block (Video Clip 7 Nerve Blocks above the Knee (Video Clip 6 The American Society of Anesthesiologists and the American College of Chest Physicians have both published guidelines for the perioperative management of patients with known or suspected obstructive sleep apnea (OSA).2,45 These guidelines highlight the association of opioids with adverse respiratory events during the postoperative period. They also stress the importance of screening patients preoperatively for signs or symptoms of OSA because up to 70% of patients who suffer from this disorder have not been diagnosed at the time of surgery.37 A common theme in all guidelines for perioperative management of OSA is the need to reduce or eliminate opioid analgesics during the postoperative period. The rate of opioid prescription in the United States has changed dramatically in response to increased pressure placed on physicians to treat pain. The Centers for Disease Control and Prevention report that the distribution of prescription opioids through the pharmaceutical supply chain increased more than 600% between 1997 and 2007.73 Since 2003, opioid analgesics have been the cause of more deaths from overdose than cocaine and heroin combined.73 This increase in opioid abuse and accidental deaths has also been a catalyst for efforts to decrease the role of opioids as the primary analgesics after surgery. Multimodal analgesia is the practice of combining more than one type of analgesic to optimize pain control while minimizing the adverse effects of individual agents.70,90,109 It is a promising way to decrease or eliminate the role of opioids in postoperative pain control. A number of pharmacologic agents can be used concurrently to provide analgesia, including nonsteroidal antiinflammatory drugs (NSAIDs), acetaminophen, gabapentinoids, mu agonists, and serotonin/norepinephrine reuptake inhibitors (Table 5-1). Peripheral nerve blocks also provide a major component in reducing opioid requirements.48 The increased availability of high-resolution ultrasound has greatly facilitated the use of peripheral nerve blocks as a component of multimodal analgesia. Nonsteroidal antiinflammatory drugs belong to broad class of medications used to treat pain and inflammation. The mechanism of action for these agents is to block the production of prostaglandins. This effect is achieved by the inhibition of cyclooxygenase. At least two isoenzymes (COX-1, COX-2) have been identified for cyclooxygenase. The COX-1 isoenzyme has been called a “housekeeping” enzyme responsible for maintaining physiologic functions of the gastrointestinal tract, platelets, and kidneys. The COX-2 isoenzyme is involved primarily in production of prostaglandins that intensify the inflammatory response.15 Nonspecific NSAIDs are effective for relieving pain and inflammation but are also associated with platelet dysfunction, gastrointestinal toxicity, renal toxicity, cardiovascular risk, and the potential for impaired bone and connective tissue healing. The introduction of COX-2–specific NSAIDs (referred to as coxibs) has been an attempt to reduce or eliminate two of these unwanted effects, namely, gastrointestinal and platelet effects.43,88 At present, celecoxib is the only COX-2–specific NSAID available for use in the United States. Other coxibs, such as rofecoxib and valdecoxib, have been removed amid concerns that they are associated with increased cardiovascular risk. Clinicians should be mindful that all NSAIDs (COX-2–specific and nonspecific NSAIDs) must be used with caution in patients at increased risk of cardiovascular disease.63,85 Gastrointestinal side effects of NSAIDs are more common in older patients, those with a prior history of peptic ulcer disease, and those receiving aspirin.91 The improved gastrointestinal tolerance with celecoxib and other COX-2–specific NSAIDs is expected because these drugs were developed in part to reduce gastrointestinal side effects. When using nonspecific COX inhibitors, however, the incidence of gastrointestinal side effects can also be significantly reduced with proton pump inhibitors.52,57 Although all NSAIDs can be associated with cardiovascular risk, the COX-2–specific drugs have been the subject of most recent reports of edema, ventricular dysfunction, and cardiac ischemia. The cardiovascular risk associated with these drugs is thought to be partly the result of a relative imbalance of prostacyclin and thromboxane.49 At present, only one COX-2 inhibitor (celecoxib) remains approved for use in the United States by the Food and Drug Administration (FDA). The early clinical evidence suggesting cardiovascular risk related to NSAIDs was largely associated with prolonged use; however, recent studies suggest that increased cardiovascular risk occurs almost immediately after initiation of therapy.85 Bone and soft tissue healing are additional concerns with the use of all NSAIDs. Reviews of in vitro and animal studies document changes in bone and soft tissue healing associated with NSAID use.16,78 Although clinical data do not consistently show outcome differences in patients treated with NSAIDs,51 there is enough evidence to justify careful consideration before their use. A reasonable approach for the use of NSAIDs in the perioperative period is to limit or avoid their use in patient populations or procedures that typically have high rates of failure or nonunion.6,75 A common question that arises with the use of celecoxib is whether it can be used safely in patients who have a history of allergy to sulfonamide antibiotics. Meta-analyses suggest there is a greater incidence of allergic reactions, in general, among patients with sulfonamide hypersensitivity; however, there is not an increased risk specific to celecoxib.72,92 Acetaminophen and its parenteral form, paracetamol, are commonly used in conjunction with opioids for postoperative analgesia. The mechanisms of action for these drugs is not well defined but has been linked to cyclooxygenase inhibition in the central nervous system (CNS) as well as central modulation of the serotonin system.13,44 Both agents are well tolerated, with relatively few side effects. Paracetamol, the intravenous formulation of acetaminophen, was approved for use in the United States in 2011. A unique application for paracetamol is for patients who cannot take oral acetaminophen. There seems to be little difference in analgesic efficacy between oral and parenteral forms of acetaminophen if therapeutic levels are achieved. However, at least two studies comparing plasma levels in surgical patients after preoperative dosing of oral acetaminophen or parenteral paracetamol show therapeutic plasma levels are reliably achieved only when intravenous paracetamol is used.17,99 In fact, less than 50% of subjects receiving oral doses achieved therapeutic plasma concentrations.17 An important aspect of multimodal analgesia that can be easily overlooked is the simple practice of combining acetaminophen (or paracetamol) with an NSAID. This approach has been evaluated in the setting of paracetamol alone versus the combination as well as NSAIDs alone versus the combination. In both situations, there were significant reductions in both pain scores and opioid requirements.55,68 An especially appealing aspect of this practice is that many of the studies showing improved pain control involved ibuprofen and acetaminophen, which are inexpensive and easily accessible by patients. Pregabalin and gabapentin are unique drugs that are structurally related to gamma-aminobutyric acid. They act by binding to voltage-dependent calcium channels in the central and peripheral nervous system and thereby alter the release of excitatory neurotransmitters.40 These actions produce anticonvulsant, antihyperalgesic, and anxiolytic effects.110 Although both drugs are used for pain control, pregabalin has some unique characteristics that make it well suited for postoperative analgesia. Compared with gabapentin, pregabalin has faster onset, more predictable plasma concentrations, and fewer side effects.40 Pregabalin has been studied in a number of perioperative settings and found to reduce opioid requirements as well as opioid-related side effects.4,14,35,97 The most commonly reported side effects of pregabalin are dizziness and somnolence. Clinical effect is achieved rapidly after oral administration of pregabalin. There is early onset of analgesia, and peak plasma concentrations are reached within 1 hour.10 Pregabalin has been studied in a range of doses as an adjunct for postoperative pain control. Although a typical starting dose is 75 mg bid, effective doses for reducing perioperative opioid requirements ranged up to 150 mg bid.58,110 A unique finding in patients treated with pregabalin in the perioperative period is a lower incidence of chronic pain associated with surgery. For example, patients treated with pregabalin after total knee arthroplasty had a significantly lower incidence of chronic neuropathic pain at 6 months compared with controls.20 Likewise, functional outcomes and pain scores at 3 months after lumbar diskectomy were improved in patients treated perioperatively with pregabalin.19 Anecdotal differences with respect to nausea, pruritus, and analgesic efficacy are a common reason for “opioid swapping” in clinical practice. However, the continued publication of reviews on the management of opioid-related side effects suggests that no particular opioid has emerged as “clearly superior” with respect to side-effect profile.8,93 Opioids such as oxycodone, hydrocodone, and hydromorphone may differ with respect to potency and dosing interval; however, they are surprisingly similar in terms of analgesic efficacy and side-effect profile when compared in equipotent doses. The most successful measures for reducing opioid-related side effects have resulted from reducing their dose requirements through multimodal analgesia.61 Tramadol and tapentadol are analgesics that act through a dual mechanism of opioid receptor activity and monoamine reuptake inhibition.67 In the case of tramadol, reuptake of both serotonin and norepinephrine are inhibited.32 For tapentadol, only norepinephrine levels are affected.38 A notable difference between tramadol and tapentadol and equipotent doses of traditional opioids is their potential for respiratory depression. These drugs do not rely on mu agonist activity as their sole mechanism for analgesia. As such, there is some evidence to suggest they may associated with less respiratory depression than conventional opioids at doses providing comparable levels of analgesia.53,76,77,87 Respiratory depression is always a concern when treating postoperative pain, and certain patient populations are especially at risk. Thus tramadol and tapentadol, while still associated with respiratory depression, may be preferable for patients at risk for obstructive sleep apnea or with other specific risk factors for respiratory depression. A unique characteristic of tapentadol is its low incidence of gastrointestinal side effects. Compared with other commonly prescribed opioids, tapentadol causes significantly less nausea, vomiting, and constipation.47,67 Thus, in patients in whom these symptoms are problematic, tapentadol may be especially advantageous. This decrease in nausea and other gastrointestinal side effects has not been observed to the same degree for tramadol. Serotonin and norepinephrine reuptake inhibition have been shown to produce analgesia even in the absence of mu receptor activity. Duloxetine, a selective serotonin and norepinephrine reuptake inhibitor, has been reported to significantly reduce opioid requirements by more than 30% after knee arthroplasty.50 In light of their effect on monoamine reuptake inhibition, caution should be used before prescribing tramadol or tapentadol in combination with other drugs that might increase CNS serotonin or norepinephrine concentrations. Serotonin syndrome is a potentially life-threatening condition that may develop in patients treated with drugs whose mechanism of action involves increased CNS serotonin and norepinephrine levels.67 This syndrome is also known to be associated with the use of this class of drugs in patients treated with opioid analgesics. Obstructive sleep apnea is a breathing disorder that is often not diagnosed until surgery. In a recent review, it was reported that more than 70% of patients with OSA were not diagnosed until their preoperative evaluation.2 Unfortunately, OSA may first be detected in the perioperative period as a result of a respiratory or cardiac complication. After surgery, OSA may manifest as hypoxemia, cardiac ischemia, delirium, and arrhythmias. Because of its association with obesity and increased age, the prevalence of OSA has increased steadily in recent years. The American College of Chest Physicians and the American Society of Anesthesiologists (ASA) have outlined treatment guidelines for screening and perioperative management of OSA patients.2,45 Their recommendations include specific aspects of the preoperative, intraoperative, and postoperative care. In the preoperative evaluation, it is important to conduct a directed history and physical examination in all patients because many with OSA are undiagnosed at the time of surgery. Specific questionnaires have been developed to screen for OSA. These include the STOP-BANG and ASA questionnaires that stratify patients as low or high risk for OSA. The STOP-BANG scoring system uses a mnemonic to present an eight-point evaluation validated by Chung et al.27 Specifically, the questionnaire identifies loud snoring (S), daytime tiredness (T), observed apnea during sleep (O), and treatment for high blood pressure (P). The other characteristics associated with OSA are body mass index (BMI) greater than 35 kg/m2 (B), age greater than 50 years (A), neck circumference greater than 40 cm (N), and male gender (G). Patients with three or more of these eight criteria are considered high risk for OSA. The intraoperative management of patients with OSA is focused on avoiding anesthetics that have a prolonged effect on recovery of spontaneous ventilation. Thus it seems reasonable to favor regional over general anesthesia and to use short-acting anesthetics whenever possible. However, despite the logic of these strategies, there are no prospective trials showing improved outcomes with any specific anesthetic technique.28 There is consensus, however, about the practice of minimizing the use of opioids in the perioperative period. Patients with OSA have an increased sensitivity to respiratory depression caused by opioid analgesics compared with controls without OSA.18,103 An important question with respect to postoperative management of OSA patients is whether they should be candidates for ambulatory surgery. There is little prospective information on outcome for OSA patients having outpatient surgery. The ASA has suggested that management of OSA patients should be based on three factors. These factors include the severity of their OSA symptoms, the scale of surgery/anesthesia performed, and the anticipated need for postoperative opioid analgesics. Only patients who have minimally invasive procedures requiring little or no postoperative opioids are considered for ambulatory surgery. Table 5-2 contains a summary of the preoperative, intraoperative, and postoperative considerations in patients at risk for OSA. Table 5-2 Perioperative Management of Patients with Obstructive Sleep Apnea Preoperative Management Screen all patients for signs/symptoms of obstructive sleep apnea (OSA) with a validated protocol such as STOP-BANG.27 Patients with a documented history of OSA or with three or more positive findings on STOP-BANG screening should be identified as high risk for OSA and informed of the likely need for hospital admission after surgery. Patients with a known history of OSA who are already treated with continuous positive airway pressure (CPAP) should bring their device from home. Intraoperative Management Where reasonable, regional anesthesia or peripheral nerve block for anesthesia and postoperative analgesia should be provided. Use short-acting anesthetics and minimize the use of opioids. Postoperative Management For Hospitalized Patients Consider nonopioid analgesia and peripheral nerve blocks wherever possible. Maintain patient in semiupright position to avoid airway obstruction (avoid supine position). Continue the use of CPAP for patients already on this therapy at home. See references 2, 27, 28, and 45. In recent years, there has been a significant increase in the number of patients treated chronically with opioids. These patients present a unique challenge in the perioperative period for several reasons. First, tolerance can develop very rapidly after initiating therapy with opioid analgesics.25,46,100 There is considerable evidence to suggest that chronic opioid use may even cause a hyperalgesic state in some patients.60 Thus, during the postoperative period, chronic opioid-consuming patients may report higher pain scores despite having higher levels of sedation when compared with opioid-naïve controls.79 In fact, the incidence of severe sedation postoperatively in opioid-tolerant patients is more than twice that of opioid-naïve controls. It has been suggested that lethal overdoses of opioid in chronic users may be due to a narrowing of the ratio between analgesic and lethal plasma concentrations.104 As with OSA patients, nonopioid analgesics and peripheral nerve blocks should be utilized wherever possible to provide improved pain control. Use caution when trying to achieve a specific “pain score.” As previously stated, chronic opioid-consuming patients typically report higher pain scores at rest and with movement despite having higher levels of sedation.79 Thus an objective measure of opioid effect, such as respiratory rate, is appropriate for maintaining safety. When dosing opioids in the postoperative period, it should be noted that opioid-tolerant patients may require significantly greater doses than opioid-naïve patients.31,71 Patients should have their preoperative opioid doses restarted as soon as it is practical. Additional analgesia should be provided ideally with nerve blocks and nonopioid analgesics. Additional opioids should be available “prn” but not as long-acting, scheduled doses. Practical considerations for the postoperative management of opioid tolerant patients are found in Table 5-3. Table 5-3 Practical Considerations for Chronic Opioid-Consuming Patients 1. Instruct the patient and family that safety is the primary goal. For that reason, respiratory rate and level of consciousness will be used to regulate opioid administration rather than by pain score alone. 2. Preoperative opioid doses should restarted as soon as is practical. Additional opioids to treat acute pain should be prescribed as immediate-release only and scheduled on a “prn” basis. 3. Schedule these patients early in the day to allow adequate time in the perioperative anesthetic care unit and in the patient’s room (if hospitalized) to observe the planned analgesic regimen and adjust as needed during normal working hours. 4. Utilize all additional elements of multimodal analgesics where appropriate, including nonsteroidal antiiflammatory drugs, pregabalin, acetaminophen (paracetamol), peripheral nerve blocks, and mixed mu agonist/monoamine reuptake inhibitors (tramadol/tapentadol). 5. Patients requiring large doses of opioids should be cared for in a monitored setting with continuous pulse oximetry and supplemental oxygen. For many years, electrical nerve stimulation (NS) was the “gold standard” technique for performing nerve blocks. Despite an absence of data showing a consistent relationship between the stimulating needle current and the distance to the nerve,12,81,82,98 NS remained popular due to the lack of any alternative method to guide needle placement. A troubling aspect of NS-guided blocks is the frequency with which they result in unintentional needle trauma and intraneural injection.82 In a recent study of sciatic nerve (SN) blocks performed using NS guidance, 94% of patients experienced unintended nerve penetration and intraneural injection.83 This high rate of intraneural injection reflects the inability to consistently produce motor response from the stimulating needle even when it is positioned on or within the target nerve.82,98 There is considerable disparity in the rate of neurologic complications reported for patients having NS-guided blocks. For example, in the previously mentioned study, where the authors described a 94% rate of intraneural injection, there were no reported neuropathies.83 By contrast, in a larger series of NS-guided SN catheters placed for foot and ankle surgery, 41% of patients reported postoperative neuropathic symptoms. Of these patients, 11% required extra hospital visits specifically for treatment of neurologic symptoms.41 Thus, although the actual incidence of nerve injury associated with NS block may vary, laboratory and clinical data confirm that needle trauma and intraneural injection are not only uncomfortable for the patient36 but injurious to the nerve as well.29,39,80,86,106 The introduction of high-resolution US has been an important development in regional anesthesia. Although initially viewed by some as a novelty or merely a supplement to NS techniques,11 US is now established as the preferred method for performing nerve blocks. Indeed, when compared with NS techniques, blocks performed using US have higher success rates, less procedure-related pain, fewer vascular punctures, fewer needle passes, lower local anesthetic requirements, and shorter performance times.1,42,59,62,64,74 Perhaps even more compelling, when NS is used in an attempt to supplement US guidance, nerve blocks take longer to perform and require more needle passes.34,89
Anesthesia
![]() )
)
![]() )
)
A Changing Role for Opioids
Multimodal Analgesia
Components of Multimodal Analgesia
Nonsteroidal Antiinflammatory Drugs
Acetaminophen and Paracetamol
Combining Paracetamol (Acetaminophen) and Nonsteroidal Antiinflammatory Drugs
Pregabalin and Gabapentin
Opioid Agonists
Combined Opioid Agonist–Monoamine Reuptake Inhibitors
Patients Who Require Special Consideration
Obstructive Sleep Apnea
Chronic Opioid-Consuming Patients
Regional Anesthesia
Ultrasound-Guided Peripheral Nerve Blocks
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine