Age
Tumor
First and second decade
Nonossifying fibroma
Unicameral bone cyst
Aneurysmal bone cyst
Chondroblastoma
Langerhans cell histiocytosis
Osteosarcoma
Ewing sarcoma
Third through fifth decade
Giant cell tumor
Lymphoma
Parosteal osteosarcoma
Fourth decade and above
Metastasis
Myeloma
Chondrosarcoma
Chordoma
Lymphoma
MFH
Table 2.2
Common tumors and tumorlike lesions by site
Epiphysis |
Chondroblastoma (open physis) |
Giant cell tumor (closed physis, subarticular) |
Osteomyelitis |
Clear cell chondrosarcoma |
Metaphysis |
Nonossifying fibroma (eccentric) |
Unicameral bone cyst (central) |
Enchondroma |
Aneurysmal bone cyst |
Osteoid osteoma |
Osteoblastoma |
Osteosarcoma |
Chondrosarcoma |
Malignant fibrous histiocytoma |
Lymphoma |
Diaphysis |
Fibrous dysplasia |
Enchondroma |
Osteofibrous dysplasia |
Adamantinoma |
Ewing sarcoma |
Periosteal osteosarcoma |
Lymphoma |
Table 2.3
Select benign entities that may be multiple at presentation
Brown tumors in hyperparathyroidism |
Enchondromatosis |
Fibrous dysplasia |
Hemangiomatosis |
Langerhans cell histiocytosis |
Nonossifying fibromas (Jaffe-Campanacci syndrome) |
Osteochondromatosis |
Paget’s disease |
SAPHO |
Imaging Modalities
Radiographs
Although various imaging modalities are available, radiographs are the mainstay and most cost-effective imaging modality for the evaluation of bone tumors. Further evaluation, if necessary, would depend on the radiographic interpretation. In some instances, lesions have such a characteristic radiographic appearance that the diagnosis can be confidently made with radiographs, and no further imaging is needed. Nonossifying fibroma and fibrous dysplasia fall into this category. Other cases warrant further workup with advanced imaging studies or tissue sampling to establish the diagnosis. Radiographs localize the lesion and also provide key discriminating factors used in lesion characterization, including lesion margins, matrix mineralization, and periosteal reaction. The pattern of destruction and margins are used to determine the aggressiveness of a lesion. A well-defined lesion with a sclerotic border is the least aggressive pattern and indicates a slow-growing benign lesion. This can also be seen with Brodie abscess. A moth-eaten pattern of bony destruction is a more aggressive pattern and is seen with malignant lesions and with osteomyelitis. A lesion with a permeative appearance, cortical destruction, and aggressive periostitis with or without a soft tissue mass is the most aggressive pattern and is seen with Ewing sarcoma, aggressive malignancies, and infection. Periosteal reaction is likewise used to predict aggressiveness and can be continuous and solid, usually seen with a benign slow-growing process, or aggressive, with laminated, hair-on-end, or sunburst appearance or producing a Codman triangle, seen with sarcomas or sometimes osteomyelitis. The matrix of a lesion is also evaluated to determine if the tumor is an osteogenic or chondroid lesion. Chondroid matrix has a characteristic rings-and-arcs or stippled appearance, as is seen in lesions such as enchondroma and chondrosarcoma. The osteoid matrix has a fluffy or cloud-like appearance and is seen in bone-forming lesions such as osteosarcoma. Matrix mineralization is usually easily identified in lesions located in the extremities; however, when subtle, computed tomography (CT) can allow for better evaluation. CT is also used to detect or characterize lesions in complex anatomic locations such as the pelvis and spine that may not be adequately evaluated with radiographs.
Computed Tomography
Computed tomography provides the best evaluation of cortical integrity, osseous remodeling, and matrix mineralization. Thin-section imaging provides fine bony detail and isotropic volume acquisition allows for high-quality multiplanar reformatting. Subtle matrix mineralization, which can sometimes be faint or undetectable on radiographs, can be seen with CT and provides evidence of lesion histology. Soft tissue extension and pathologic fractures that may not be radiographically apparent can also be seen on CT. CT is particularly useful for determining the presence of and evaluating the extent of lesions in locations that may not be seen on radiographs because of complex anatomy, particularly in the pelvis, spine, ribs, scapula, and chest wall. In other locations, magnetic resonance imaging (MRI) is most often used as the next imaging test as well as for staging. CT however is the examination of choice when osteoid osteoma is in the differential diagnosis, because this imaging modality reliably demonstrates the nidus, permitting a confident diagnosis and serving as the basis for planning radiofrequency ablation. CT is also the imaging modality used most frequently for biopsy guidance and to evaluate the lungs for metastasis. Because of hematologic spread, the lungs are the most common site of metastasis from primary sarcomas of the bone, and with high-grade sarcomas, most patients will have pulmonary micrometastasis at presentation.
Magnetic Resonance Imaging
Magnetic resonance imaging is the examination of choice for the staging of bone tumors and is critical in planning limb salvage and tissue-sparing surgical procedures. This imaging technique provides the best evaluation of marrow involvement and, because of exceptional contrast resolution, the best evaluation of the soft tissue extent of a tumor, including the relationship to neurovascular structures. Fluid, necrosis, and hemorrhage are also best seen on MRI. An additional benefit of this modality is that ionizing radiation is not used. However, because of chemical shift artifact, MRI provides suboptimal evaluation of the presence and extent of cortical involvement and is not very sensitive for the detection of matrix mineralization. These features are best evaluated with CT. MRI should always be performed before biopsy and can direct the biopsy to solid components of a lesion, which should be sampled to avoid a false-negative result from cystic or necrotic areas of tumor. Typical pulse sequences include multiplanar T1 and fat-saturated T2 fast spin-echo images, with coil selection for optimal signal-to-noise ratio and spatial resolution sufficient to cover the anatomic area of interest. These sequences provide comprehensive information for accurate staging, and we do not believe that gadolinium-enhanced contrast studies are routinely required for the staging of bone neoplasms. An important principle to remember is that when sarcoma is considered, the field of view should include the entire bone, including the adjacent joints, to evaluate for skip lesions that may not be detected with bone scintigraphy and for possible joint involvement. Another factor that is important for surgical staging of malignant lesions is that the entire extent of edema-like signal is considered the reactive zone which should be removed along with a rim of uninvolved adjacent tissue. Although MRI is sensitive for lesion detection and can characterize some lesions based on their appearance, it is not specific and necessitates correlation with radiographic features to arrive at a diagnosis. Discordance between radiographs and MRI usually occurs with benign lesions that have a propensity to produce edema-like signal well beyond the confines of the tumor. Lesions that frequently demonstrate this appearance are the following: chondroblastoma, osteoid osteoma, LCH, solid ABC, and Brodie abscess. In general, it is important to remember that in the absence of fracture, profound edema-like signal is an exceptional finding with sarcomas of the bone. Some neoplasms that are frequently encountered with normal radiographs but abnormal MRI are lymphoma, metastasis, and myeloma. It is exceptionally rare for a sarcoma of the bone to be discovered on MRI with normal radiographs. MRI can be used for treatment follow-up, to evaluate response to therapy and to assess for possible recurrence. When residual or recurrent tumor is suspected, contrast-enhanced sequences are of value.
Bone Scintigraphy
Bone scintigraphy uses Tc99m-labeled diphosphonates that bind to hydroxyapatite at sites of osteoblastic activity, resulting in increased radionuclide uptake at areas of bone turnover. This uptake is nonspecific and occurs in many benign and malignant bone tumors along with fractures, osteomyelitis, and other nonneoplastic conditions. Abnormal bone scans should therefore always be correlated with clinical information and other imaging studies. Scintigraphy is much more sensitive than radiography and even CT and can identify abnormalities that are not visualized by these imaging modalities. In the realm of bone tumor imaging, scintigraphy is used to confirm that a lesion is solitary when first identified on a radiograph and is most consistently performed to determine the distribution of metastasis when this is a consideration based on the initial lesion. Scintigraphy does not effectively evaluate the intraosseous or soft tissue extent of a lesion. Although malignant lesions generally tend to have greater activity than active benign processes, scintigraphy alone cannot be used for discrimination and should be correlated with radiographs. For example, fibrous dysplasia and Paget’s disease are benign entities that show avid uptake. Giant cell tumor can have increased uptake about the adjacent joint caused by hyperemia and disuse osteoporosis, not from tumor extension. Tumors such as LCH or chondrosarcoma may not show increased uptake, and lymphoma, myeloma, and highly anaplastic tumors often show no uptake on bone scan. Purely osteolytic tumors such as metastasis from renal cell or thyroid cancers will show photopenic areas. Bone scintigraphy is also used to follow response to therapy; however, positron emission tomography (PET)-CT is gaining favor for this purpose.
Positron Emission Tomography-Computed Tomography
On a consistent basis, the most valuable role of 18fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT is for systemic disease staging, evaluating the skeleton and lungs for the presence of metastasis, and evaluating the response to neoadjuvant therapy. PET uses a glucose analog labeled with fluorine-18 that is administered intravenously to detect lesions with increased metabolic activity. These images are fused with those from CT to improve lesion detection and characterization, including the detection of subcentimeter lung nodules that would otherwise not be detected with PEt alone because of their small size. A large retrospective review comparing bone scintigraphy and 18FDG PET-CT for the detection of bone metastasis in osteosarcoma found that PET-CT was more accurate and sensitive for the detection of osseous metastasis. In patients with Ewing sarcoma, PET-CT has also been found to allow for more accurate staging than PEt alone. Given that PET-CT is commonly used for staging many cancers and lymphoma, it is important to be aware that some benign bone lesions, including enchondroma and fibrous dysplasia, are FDG avid, which could result in false-positive results if not correlated carefully with the CT portion of the exam. If the diagnosis is not apparent on CT, further evaluation with radiographs may be warranted. Other potential causes of false-positive results are fracture and infection. In contrast, chondrosarcoma, a malignant bone tumor, may be mistaken for a benign lesion based on its low to moderate uptake (depending on the grade of the lesion).
Staging
The major contribution of imaging in the management of malignant bone tumors is local and systemic staging. Local staging is most consistently achieved with MRI to determine the intracompartmental extent as well as the presence of extracompartmental disease and its relationship to neurovascular structures. Systemic staging has largely relied on CT of the thorax and scintigraphy but is gradually being replaced by PET-CT.
Image-Guided Biopsy
Image-guided percutaneous or open surgical biopsy is often required to obtain the histological diagnosis of an indeterminate or aggressive bone lesion. Percutaneous needle biopsy of bone lesions has been shown to be a safe and accurate method with a very low (1.1 %) complication rate. CT has replaced fluoroscopic guidance in most institutions as the modality of choice, often with the use of low-dose techniques to limit radiation exposure. Nearly all primary and metastatic bone tumors are visible on CT, which allows for accurate needle positioning within the lesion. In very rare cases, however, bone marrow lesions may not be visualized on CT despite obvious findings on MRI or PET. In these cases, MRI can be a safe and reliable alternative for guiding the biopsy which requires MR-safe equipment and expertise. Because metastasis and myeloma are vastly more common than primary bone tumors, complete workup before biopsy of a lesion is extremely important, as this can lead to the diagnosis of a metastatic lesion thought to be a primary bone tumor. Additionally, when multiple lesions are present, a complete workup before biopsy will allow detection of the most easily accessible site for biopsy. MRI should always precede the biopsy.
The single most important issue to be understood by the radiologist performing these procedures is compartmental anatomy. Most treating surgeons remove the biopsy track, so it is critical for the radiologist performing the procedure not to compromise the definitive surgical procedure by approaching the lesion through an inappropriate compartment. Discussing the biopsy approach with the surgeon who will be carrying out the definitive operation would clarify all issues before an irreversible biopsy approach is taken. The details of needles and techniques are beyond the scope of this chapter.
Image-guided treatments have become popular since the introduction of radiofrequency ablation of osteoid osteomas in 1992 by Dr. Rosenthal. Nowadays, many primary and metastatic bone and soft tissue tumors are successfully treated or palliated by a variety of image-guided ablation techniques, including radiofrequency ablation, microwave ablation, and cryoablation.
Characteristically Benign Lesions
There are several benign lesions with such a radiographically classic appearance that biopsy or further imaging would be inappropriate. These commonly encountered lesions would include in the pediatric and adult population some of the following.
Nonossifying Fibroma
Nonossifying fibroma (NOF) is a benign fibrous lesion composed of spindle-shaped fibroblasts in a whorled pattern with scattered giant cells, foam cells, and small amounts of collagen. The lesion is commonly seen in children and adolescents, with a reported incidence of 30–40 % prior to skeletal maturity and a male to female predominance of 2:1. Lesions can be solitary or multiple and are most often located in the long bones of the lower extremities, commonly the distal femur and tibia, with lesions in the upper extremities and elsewhere being uncommon. Nonossifying fibromas occur in the metaphyseal region and can extend into the diaphysis as the bone lengthens with age. Smaller lesions measuring less than 2–3 cm having the same pathology but centered only in the cortex are called fibrous cortical defects. Because of spontaneous healing that occurs with sclerosis filling in from the periphery with complete regression, it is rare to see nonossifying fibromas in adults. These lesions are asymptomatic unless complicated by a pathologic fracture and are often discovered incidentally on radiographs or MRI performed for trauma or other unrelated indications.
The fibrous nature of this lesion results in the characteristic radiographic appearance of an eccentrically located, cortically based, mildly expansile radiolucent lesion with a thin sclerotic margin and some extension into the medullary space (Fig. 2.1). Larger lesions may be multiloculated or internally septated with a soap bubble appearance (Fig. 2.2). Regardless of lesion size, there should be no significant periosteal reaction. When this classic appearance is seen, no further imaging is indicated. MRI will demonstrate a cortically based lesion with variable T1 signal intensity depending on the stage of healing, generally isointense to muscle, with corresponding low more often than high T2 signal intensity and a sclerotic low-signal-intensity rim on both T1- and T2-weighted imaging. The cortex may appear focally permeated, but there should be no associated soft tissue mass. Depending on the stage of healing, these lesions can show mild increased uptake on bone scan and may be FDG avid.
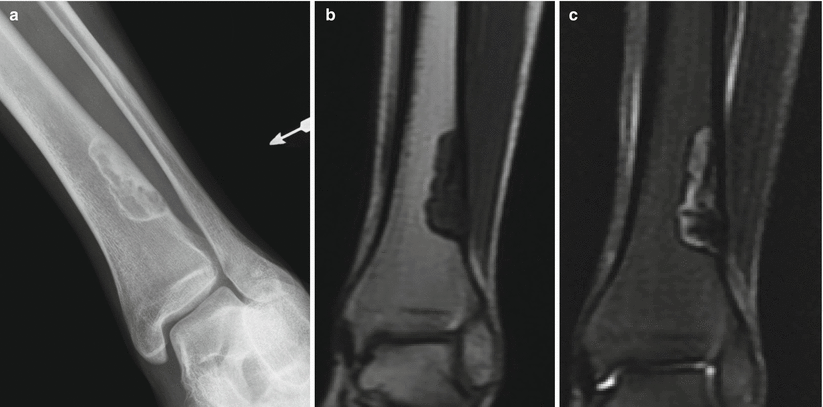
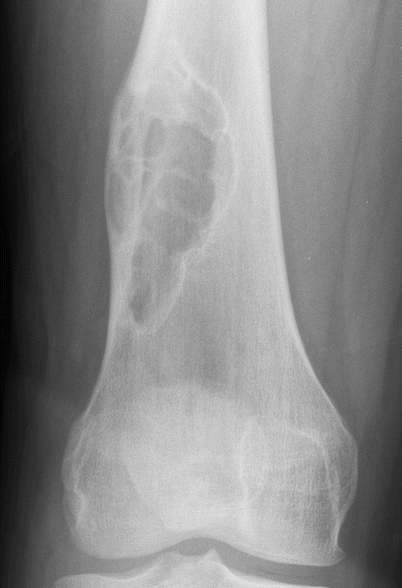

Fig. 2.1
NOF of the distal tibia in an 18-year-old man. Anteroposterior radiograph (a) shows an eccentrically located well-defined radiolucent lesion with thin sclerotic border and few internal septations. The lesion extends from the cortex into the medullary canal. There is no periostitis. Corresponding coronal T1-weighted (b) and T2-weighted (c) MR images show a sharply defined low-T1- and heterogeneous T2-signal-intensity lesion with a low-signal-intensity rim. Nonossifying fibroma is a radiographic diagnosis and does not require MRI. The MR images are shown because radiologists should be comfortable in making the diagnosis when these lesions are incidentally identified on MRI despite the variable signal characteristics, which are a manifestation of the histologic composition and stage of healing

Fig. 2.2
NOF of the distal femur in a 19-year-old man. Anteroposterior radiograph shows a larger well-defined eccentrically located expansile lesion with multiple septations typical of nonossifying fibroma. The small amount of periostitis along the lateral cortex superiorly is from a pathologic fracture
Nonossifying fibromas have no malignant potential, and no treatment is needed unless there is a risk of pathologic fracture. Multiple NOFs can be familial or can occasionally be seen with neurofibromatosis. The association of multiple NOFs and café au lait spots has been termed Jaffe-Campanacci syndrome. Severe cases can have mental retardation, cardiovascular and ocular abnormalities, and cryptorchidism.
Fibrous Dysplasia
Fibrous dysplasia is a nonhereditary benign disorder of bone remodeling that results in marrow replacement by fibro-osseous tissue. There is no gender predominance, and lesions, which are intramedullary, can be detected at any age. Fibrous dysplasia can be monostotic or polyostotic, with monostotic fibrous dysplasia being six to ten times more common. Monostotic disease does not evolve into polyostotic fibrous dysplasia, and lesions generally remain stable in size after puberty. Monostotic lesions are often incidentally discovered before the age of 30 and most commonly involve a rib, the femur (particularly the proximal end), the tibia, or craniofacial bones. Fibrous dysplasia is the most common benign rib lesion. Polyostotic lesions vary in number, can be unilateral, involving several bones of one extremity or one side of the body, or can be bilateral. Lesions tend to be larger becoming apparent during childhood because of the weakened bone, leading to limb deformities with bowing, pain, and fractures. One such example is the “shepherd’s crook” deformity of the hip with coxa vara and bowing of the diaphysis. Pathologic fractures are not uncommon and most commonly occur in the proximal femur. In the setting of a femoral lesion, the ipsilateral pelvis should be closely scrutinized for involvement because of this close association. Polyostotic lesions commonly involve the pelvis, femur, tibia, upper extremities, ribs, craniofacial bones, spine, and shoulder girdle. Various endocrinopathies can occur with polyostotic FD; the best known is McCune-Albright syndrome, which includes polyostotic fibrous dysplasia, precocious puberty, and café au lait macules. The rare association of fibrous dysplasia and benign soft tissue myxomas is called Mazabraud’s syndrome. It is important to be aware of this association so as not to confuse a myxoma for sarcoma or malignant transformation of fibrous dysplasia.
Radiographically lesions can have several different patterns depending on the amount of bony trabeculae and fibrous elements and can have a lucent, mixed, or sclerotic appearance. The classic appearance in a long bone is a mildly expansile, well-circumscribed intramedullary lucent lesion in the diaphysis or metadiaphysis with a ground-glass appearance and often with a sclerotic border (Figs. 2.3 and 2.4). Lesions can have cortical scalloping, but there should be no cortical disruption or periosteal reaction unless there is a pathologic fracture or malignant transformation. Lesions, particularly in the ribs, but also elsewhere, can show extensive expansion and deformity (Fig. 2.5). Craniofacial lesions are often unilateral and have a ground-glass or mixed sclerotic appearance because of the large osseous component with bony expansion, which can lead to neurologic dysfunction or exophthalmos. Leontiasis ossea is a rare facial deformity resulting from involvement of the frontal and facial bones with the deformity resembling a lion’s face. On MRI, lesions have variable signal intensity depending on the composition of the lesion, showing low to intermediate T1 signal and low, intermediate, or high signal intensity on T2-weighted imaging (Fig. 2.6). Enhancement is variable. MRI can also be used to detect fluid-fluid levels from secondary aneurysmal bone cysts. Bone scintigraphy is useful to detect polyostotic disease and shows increased uptake on all three phases.
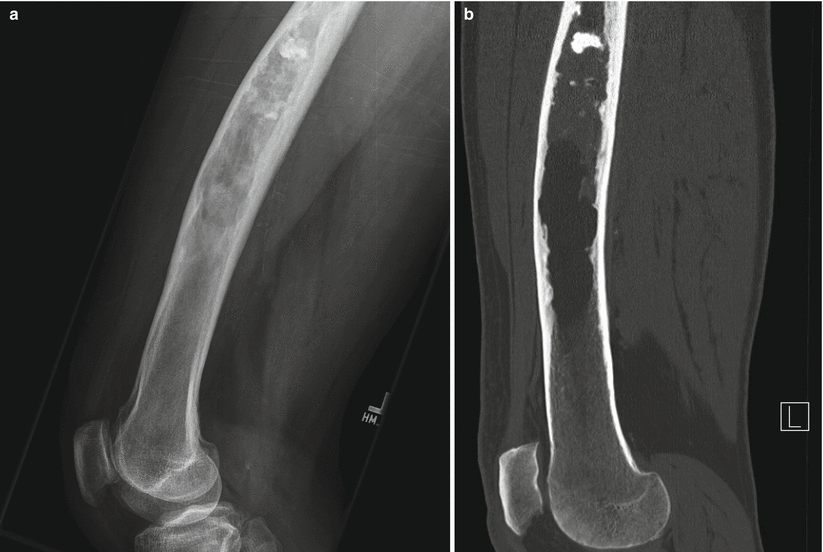
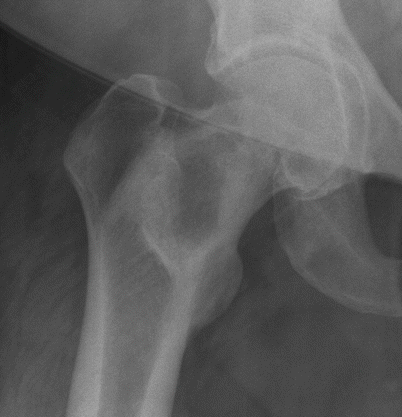
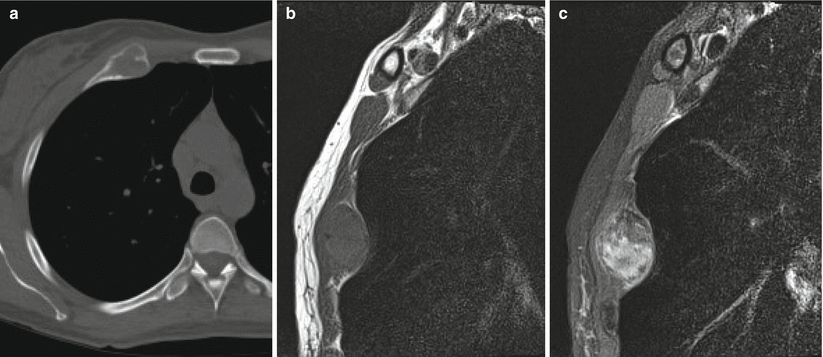
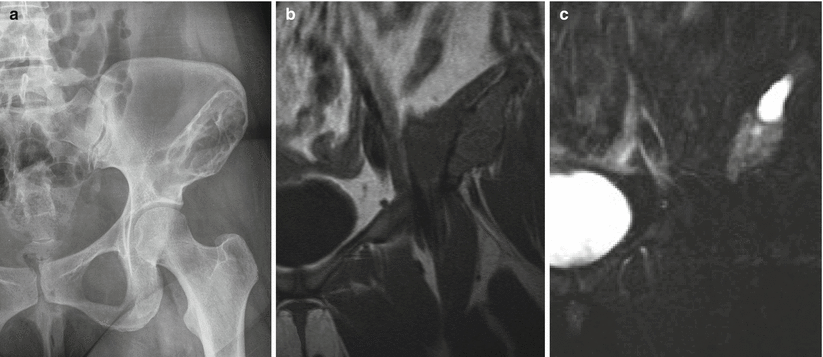

Fig. 2.3
Fibrous dysplasia of the femur in a 65-year-old man. Lateral radiograph (a) shows anterior bowing of the femoral shaft and a long intramedullary diaphyseal radiolucent lesion with expansile remodeling and endosteal scalloping with hazy “ground-glass” appearance and some focally calcified matrix. Corresponding CT image (b) shows similar findings, with some calcified matrix proximally and fat attenuation distally

Fig. 2.4
Fibrous dysplasia of the proximal right femur in a 52-year-old woman. Frontal radiograph of the right hip shows a well-defined osteolytic lesion with a thick sclerotic rim

Fig. 2.5
Fibrous dysplasia of the anterior right rib in a 42-year-old woman. Axial CT image (a) shows expansile remodeling with cortical thinning and ground-glass matrix. Sagittal T1-weighted (b) and fat-saturated T2-weighted (c) MR images show an expansile lesion with low T1 and heterogeneous T2 signal. The most common benign rib lesion is fibrous dysplasia

Fig. 2.6
Fibrous dysplasia of the left ilium. Frontal radiograph of the pelvis (a) shows a large trabeculated radiolucent lesion with a sclerotic border in the left ilium extending to the superior acetabulum. Coronal T1-weighted (b) and fat-saturated T2-weighted (c) MR images show the expansile nature of the lesion. The cortex is not breached, confirming that the lesion is entirely intracompartmental. The cyst-like bright T2 signal superiorly is a finding that can be encountered in fibrous dysplasia
Malignant transformation is rare, occurring in 0.4–1 % of cases of both monostotic and polyostotic fibrous dysplasia. Clinically increasing pain is worrisome, with imaging findings of lesion enlargement, cortical destruction, and soft tissue mass. Osteosarcoma and fibrosarcoma are the most common malignancies. Although fibrous dysplasia has a characteristic appearance, a lesion that can present with similar imaging characteristics is low-grade central osteosarcoma. The discriminating factor is cortical destruction, which should not be present with fibrous dysplasia.
Enchondroma
Enchondromas are benign intramedullary chondroid lesions that are thought to occur as a result of ectopic cartilage rests arising from displacement of the growth plate. These lesions are usually solitary and are the second most common benign chondroid tumor after osteochondroma. Although the true incidence is unknown due to their largely incidental discovery, they account for at least 3 % of all bone tumors. Enchondromas have been seen in all age groups, but the peak incidence is in the third and fourth decades. There is no gender predominance. Lesions are most commonly located in the metaphysis or metadiaphysis of the appendicular skeleton, with the phalanges of the hands being the most common site; enchondromas are the most common bone tumor of the hands. The long bones are also commonly involved, predominantly the femur and proximal humerus.
Imaging characteristics vary depending on location. In the short tubular bones of the hands and feet, there is a classic expansile lucent appearance with cortical thinning that is commonly significant and often only a small amount of punctuate chondroid calcification (Fig. 2.7). In Fig. 2.8, a third metatarsal enchondroma with a greater amount of chondroid calcification is shown. In this location, these lesions are frequently discovered because of pathologic fracture. In the long bones, where lesions are most often incidentally found, the characteristic appearance is that of an intramedullary lesion with dense chondroid calcification, having a “popcorn” or “rings and arcs” appearance without a discrete sclerotic rim or underlying lucency (Figs. 2.9 and 2.10). Lesions can be entirely calcified. Larger lesions can show cortical scalloping but should not demonstrate cortical disruption. Less commonly, the appearance can be that of a well-circumscribed lucent lesion with a thin sclerotic rim and faint scattered calcification. When the classic appearance is seen, further imaging is unnecessary and conservative follow-up is suggested. Infarcts in long bones can sometimes have a similar radiographic appearance but should not show endosteal scalloping and often show peripheral serpentine calcification. CT will show findings similar to radiographs but with better evaluation of matrix calcification as well as the depth and extent of endosteal scalloping. MRI shows a lobulated lesion with low to intermediate T1 and high T2 signal intensity because of the high water content of the hyaline cartilage, with chondroid calcifications remaining low signal on both T1- and T2-weighted images. On the T1-weighted images of enchondromas in the long bones, normal-appearing marrow fat can be seen between the cartilage lobules. Lesions show increased uptake on Tc99 MDP bone scans and this should not be used as an indicator of malignancy. Imaging features that indicate malignant degeneration are lesions that change over time with increased lucency and resorption of the internal calcifications or those that show cortical disruption or soft tissue mass. The distinction between enchondroma and low-grade chondrosarcoma can sometimes be difficult when there is extensive and deep cortical scalloping. This will be discussed further in the section dealing with chondrosarcoma.
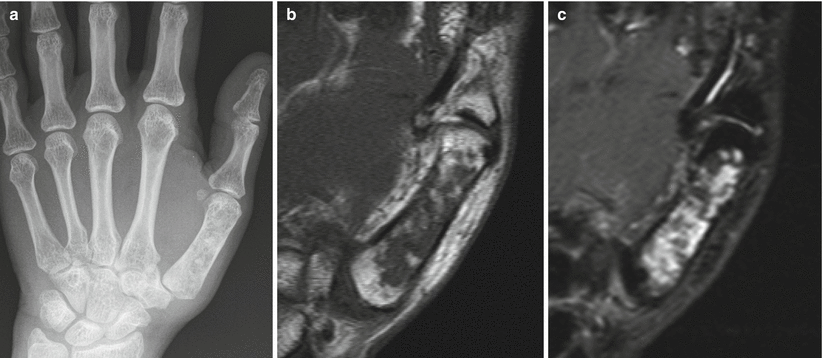
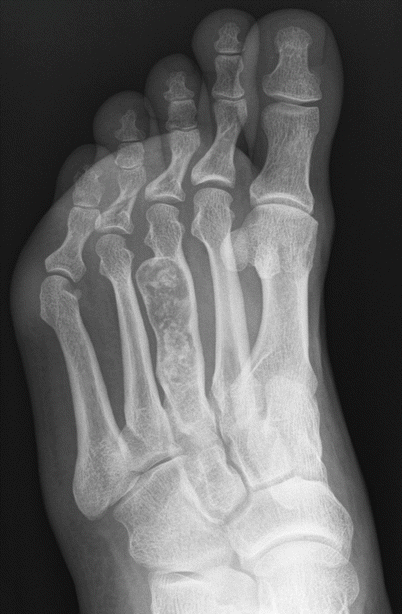
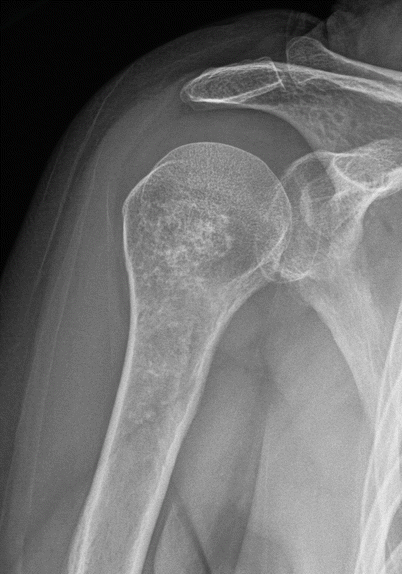
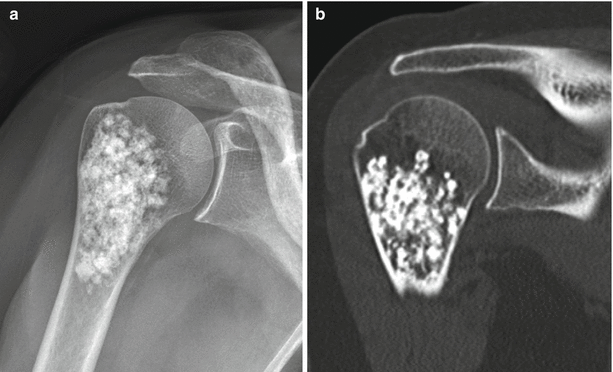

Fig. 2.7
Enchondroma of the first metacarpal in a 54-year-old woman. Frontal radiograph (a), coronal T1-weighted (b), and fat-saturated T2-weighted (c) MR images show an intramedullary lucent lesion with chondroid calcification occupying the entire metacarpal shaft with typical MR appearance of a chondroid lesion. Despite the large size of the lesion, because there is no cortical breakthrough or soft tissue mass, this appearance is consistent with an enchondroma. Primary chondrosarcoma of the short tubular bones is an exceptionally rare diagnosis

Fig. 2.8
Enchondroma of the third metatarsal. Oblique radiograph shows an expansile intramedullary radiolucent lesion in the third metatarsal shaft with chondroid calcification. The location and appearance of the lesion make for the diagnosis

Fig. 2.9
Proximal humeral enchondroma in a 45-year-old man. Anteroposterior radiograph shows an intramedullary lesion with stippled chondroid calcifications in the proximal humerus consistent with enchondroma, at a common site. These lesions are often incidentally discovered on chest radiographs

Fig. 2.10
Proximal humeral enchondroma in a 50-year-old woman. Frontal radiograph (a) shows a lobulated intramedullary lesion with dense popcorn-like calcification, typical for a heavily calcified enchondroma. Corresponding CT scan (b) shows the heavily calcified enchondroma. There is no cortical disruption, a critical negative finding
Multiple enchondromatosis is nonhereditary, has variable severity, typically shows unilateral involvement of the limbs, and has an increased risk (10–25 %) of malignant transformation to chondrosarcoma (Fig. 2.11). Ollier’s disease is a dysplasia of any portion of endochondrally formed bone with multiple enchondromas and shortened and bowed bones. Maffucci syndrome is the association of multiple enchondromas and soft tissue hemangiomas, with greatest involvement of the hands and feet and the highest risk of malignant transformation.
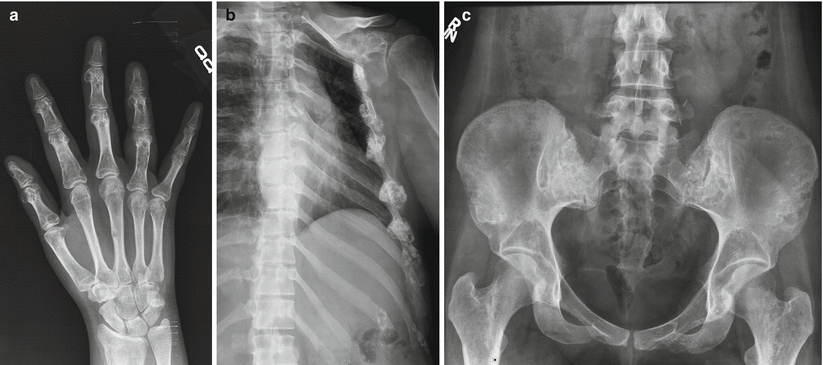

Fig. 2.11
Ollier’s disease in a 29-year-old woman with multiple enchondromas involving the hands (a) ribs, left scapula (b) pelvis, and proximal femora (c) ribs, left scapula, pelvis, and proximal femora
Enchondromas located in the epiphysis are known to occur with multiple enchondromatosis but can also occur in isolation. The appearance typically is that of a lucent lesion with well-defined borders and matrix mineralization that can be extensive. In a large series of solitary epiphyseal enchondromas, lesions were found most commonly in the proximal humerus and femur. In this series, 55 % of the lesions were eccentrically located, and the majority extended to the metaphysis and/or the subchondral bone.
Paget’s Disease
Paget’s disease is a disorder of abnormal bone remodeling that is common in older adults of European descent, occurring in 3 % of the population over age 40 and in 10 % of the population over age 80. The etiology is unknown, but the most widely supported hypothesis is that of a slow viral infection. There is a slight male predominance and a geographic predilection, with the disease commonly seen in the United Kingdom, parts of Western Europe, Australia, New Zealand, and the United States. It is distinctly rare in China and most of Africa. Any bone in the body can be involved, but the axial skeleton, particularly the lumbosacral spine and pelvis, is affected most often; the skull and proximal femur are additional common sites. The disease can be monostotic or, more frequently, polyostotic, with variable involvement that is commonly progressive with age. The disease is one of excessive osteoclastic resorption followed by a disorganized osteoblastic response, resulting in larger bones with weaker than normal bony structure that predisposes the patient to insufficiency fractures. Clinically, there can be pain with osseous enlargement and bowing, most commonly lateral bowing of the femur or anterior bowing of the tibia. Because of the weakened subchondral bone, osteoarthritis occurs at an accelerated rate in the adjacent joint, often with concentric narrowing at the joint space without significant osteophyte formation. Acetabular involvement can result in acetabular protrusion. When there is cranial or vertebral involvement, neurologic symptoms may occur, caused by bony enlargement with resulting neural foraminal impingement. Basilar invagination occurs in as many as 30 % of patients when the skull base is involved. Because of the increased osteoblastic activity, the serum alkaline phosphatase level is elevated, and because of the osteolysis, there is also an elevation in urine hydroxyproline level. The disease is also frequently discovered incidentally during imaging performed for other indications, and in uncomplicated disease because of the characteristic appearance, no imaging other than radiographs is indicated.
The disease progresses through three well-known phases, with imaging characteristics unique to each stage. In the first phase, there is excess osteoclastic resorption and replacement of the bone with fibrovascular marrow. The characteristic finding in the skull is a large, well-defined osteolytic lesion involving both the inner and outer table, known as osteoporosis circumscripta. In the long bones, the disease begins at the epiphysis and appears lytic, extending to the metadiaphysis with an advancing well-demarcated v-shaped line between the affected and normal bone; this is characteristically described as the “blade of grass” appearance. The exception to this rule is disease occurring in the tibia, where the process can begin in the tibial tubercle (Fig. 2.12). Next, there is a mixed lytic and blastic phase, with filling in with disorganized woven bone with trabecular and cortical thickening. Finally, an osteoblastic phase occurs, with continued cortical thickening and enlargement of the bone (Figs. 2.13 and 2.14). The skull takes on a “cotton wool” appearance, and in the spine, the affected vertebra is sclerotic, seen as either a picture frame or an “ivory vertebral body.” The common differential for the ivory vertebral body is metastasis or lymphoma; however, a distinguishing feature of Paget’s disease is enlargement of the vertebral body, which can also involve the posterior elements. The sclerotic phase of the disease can be confused with other disease processes such as diffuse metastasis or myelofibrosis. Metastasis can be differentiated by more widespread involvement and lack of bony expansion with trabecular thickening. Myelofibrosis will likewise not demonstrate bony enlargement and will show hepatosplenomegaly. The highest incidence of pathologic fractures occurs in the osteoblastic phase, with fractures of the long bowed bones, commonly on the convex side, seen as one or more cortical lucencies known as “banana fractures” or a complete fracture. Tc99m MDP bone scans show marked uptake with active disease and can be used to evaluate disease extent and to identify lesions before they become radiographically evident. CT shows findings similar to those seen on radiographs, with cortical and trabecular thickening and bony enlargement. MRI shows low T1 signal in areas of sclerosis with marrow fat in areas of inactive disease and heterogeneous signal with active disease. Even with active disease, interspersed fatty marrow should be seen. When there is confusion as to the diagnosis during the resorptive phase, this preservation of fatty marrow serves as an indicator of the benign nature of the disease. T2-weighted images show low signal corresponding to areas of sclerosis and fat signal intensity in areas of inactive disease, with heterogeneous intermediate signal corresponding to active disease. If the normal marrow fat is replaced, this should raise concern for neoplasm or edema from fracture.
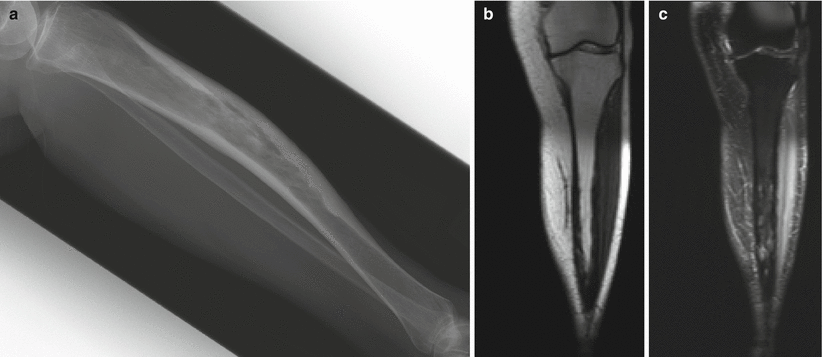
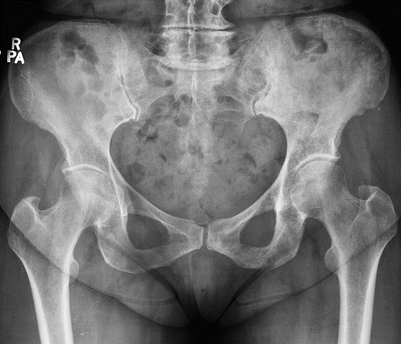
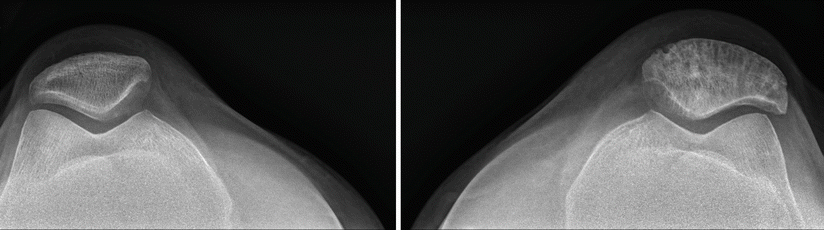

Fig. 2.12
Paget’s disease of the tibia. Lateral radiograph (a) shows anterior bowing of the tibia with cortical thickening and trabecular coarsening. Note that the tibia is the exception to the rule that Paget’s disease must start at the end of the bone. T1-weighted coronal (b) and fat-saturated T2-weighted coronal (c) MR images show low-signal-intensity cortical thickening. In this stage of inactive disease, the fatty marrow is preserved on the T1-weighted sequence, whereas there is heterogeneous increased intramedullary T2 signal, again with low-T2-signal cortical thickening. Paget’s disease is perhaps the only disease that we are aware of in which there can be extensive radiographic abnormality, as in this case, and yet the fatty marrow signal is preserved on the T1-weighted sequence

Fig. 2.13
Paget’s disease of the left hemipelvis in a 65-year-old man. Frontal radiograph shows typical findings of cortical thickening and trabecular coarsening involving the left hemipelvis

Fig. 2.14
A different patient with Paget’s disease of the left patella
Sarcomatous transformation occurs in approximately 1 % of patients with monostotic disease and in up to 10 % with polyostotic disease. The sarcomas generally occur after the age of 50; are most common in the femur, pelvis, and humerus; and are high grade, mostly osteosarcoma. The prognosis is uniformly poor. Pain and swelling may herald neoplastic transformation, which will be seen as lytic areas of bony destruction with cortical involvement and soft tissue mass. There have been cases of profound rapid spontaneous osteolysis involving bones affected by Paget’s disease after immobilization and hip fracture fixation. The osteolysis can occur several months postoperatively and it is important to be aware of this entity so as not to misinterpret it as neoplasm or infection.
Osteogenic Lesions
Tumors in this category are distinguished by their production of osteoid and are separated into benign and malignant categories.
Osteoid Osteoma
Osteoid osteoma is a benign neoplasm that represents approximately 3 % of all primary bone tumors. The actual lesion is a small oval nidus composed of variably mineralized osteoid trabecula surrounded by vascular fibrous connective tissue. The lesion has been found in patients from the age of 8 months to 70 years but is most often seen between the age of 10 and 30, with a male to female ratio of 2:1 to 4:1. Patients most commonly present with dull or aching pain that is worse at night and is often relieved by aspirin and other nonsteroidal anti-inflammatory drugs. Occasionally, with superficial lesions in the radius, anterior tibia, fingers, or toes, the presenting symptom is painless swelling or a mass. When there is involvement of the spine, scoliosis is commonly seen from muscle spasm, with the lesion located on the concave side of the curvature.
Lesions are categorized as cortical, cancellous (medullary), or intra-articular depending on the location of the nidus, with each having a characteristic pattern of associated sclerosis. The most common type is a small, cortically based nidus in the center of dense surrounding reactive sclerosis. Although any bone can be involved, cortically based lesions are most commonly found in the diaphysis or metadiaphysis of the femur and tibia. The characteristic radiographic appearance of the cortical osteoid osteoma is that of a small lucent nidus usually less than 1.5–2 cm in size surrounded by a thick reactive rim of sclerosis (Fig. 2.15). Sometimes the nidus can be calcified or the sclerosis can be so dense that it obscures the nidus. In these cases, and to help differentiate an osteoid osteoma from a stress fracture or Brodie abscess, which can have a similar appearance, CT should be performed. CT is the examination of choice because it reliably demonstrates the nidus, permitting a confident diagnosis and serving as the basis for planning radiofrequency ablation. With osteoid osteoma, CT will show the lucent round nidus with or without central mineralized osteoid rather than a more linear fracture line or a linear serpentine sinus tract that can sometimes extend to the nearest growth plate in the case of Brodie abscess. The next most common lesion type is an intramedullary nidus, which has only a small amount of eccentric sclerosis, most often located away from the nidus (Fig. 2.16). These lesions are most frequently seen in the femoral neck, posterior elements of the vertebra (particularly the lumbar spine), and small bones of the hands and feet. The least common type is the subperiosteal nidus, which displays no surrounding sclerosis or cortical thickening. These lesions are most often found along the medial aspect of the femoral neck and in the hands and feet, particularly the talus. The typical appearance is that of a juxtacortical radiolucent or sclerotic nidus with extrinsic erosion of the adjacent cortex. Lesions that are intra-articular often present a diagnostic challenge. These most often involve the hip, are often medullary or subperiosteal, and can be difficult to see on radiographs as there is little or no adjacent sclerosis depending on the distance from the joint space. Periosteal reaction can be seen where the periosteum blends with the joint capsule and may be extracapsular. Additionally, the nidus may not be seen with any imaging modality at the onset of pain and may only be seen when imaging is repeated months after the onset of symptoms. Regional osteoporosis, joint effusion, and synovitis may be present, with symptoms mimicking a synovial inflammatory process or arthritis. CT is the imaging modality of choice for lesion detection and is particularly useful for sites such as the axial skeleton, where lesions may not be seen on radiographs. Bone scintigraphy is also a sensitive imaging modality for the detection of osteoid osteoma and can be particularly useful when initial radiographs are negative or symptoms are atypical. Bone scans will show increased uptake, sometimes with a “double density sign” (a central focus of increased uptake corresponding to the nidus surrounded by a less intense area of uptake). On MRI, the nidus has intermediate to low T1 signal and variable T2 signal intensity. If the nidus is totally calcified, it will have low signal on all sequences. Enhancement is variable but commonly will be diffuse. The nidus can be difficult to detect on MRI and occasionally only perilesional edema is seen. It is important to be aware of the marked adjacent bone marrow and soft tissue edema that is commonly seen so as not to misdiagnose this lesion on MRI.
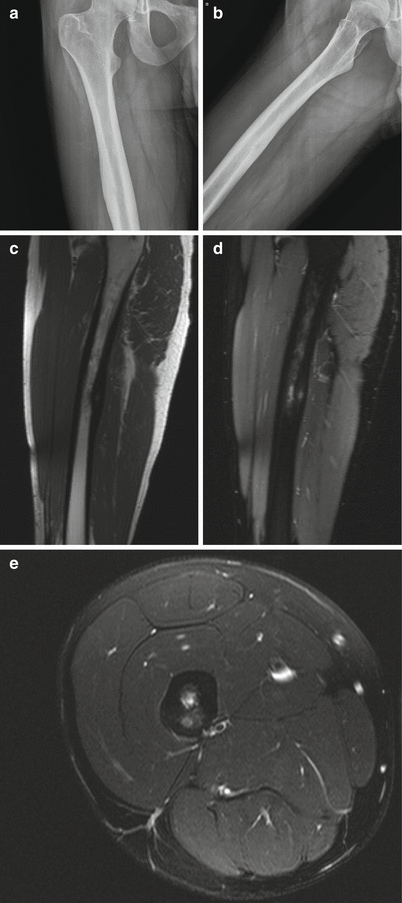
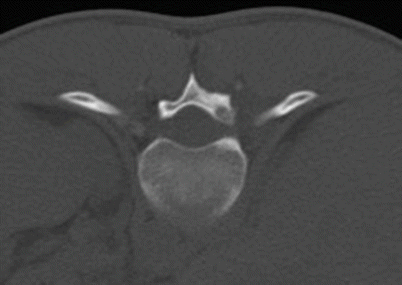

Fig. 2.15
Osteoid osteoma of the femoral shaft in a 22-year-old man. Frontal radiograph of the right femur (a) shows a smooth area of cortical thickening along the femoral shaft. Oblique radiograph (b) shows a small lucent nidus, suggestive of an osteoid osteoma. Sagittal T1-weighted (c), sagittal fat-saturated T2-weighted (d), and axial fat-saturated T2-weighted (e) MR images show the small, round, cortically based nidus surrounded by cortical thickening. Note the surrounding edema on the sagittal and axial T2-weighted sequences, a common accompanying finding with this lesion

Fig. 2.16
Osteoid osteoma in the right T11 lamina in an 18-year-old man. Axial CT image shows the lucent intramedullary nidus in the T11 lamina with a tiny area of central sclerosis and a small amount of eccentric sclerosis posteriorly
Although osteoid osteomas can resolve spontaneously, pain and gastrointestinal symptoms related to long-term NSAID use often warrant invasive treatment. The current standard of care for treatment is CT-guided radiofrequency ablation, which has a high success rate and minimal morbidity (Fig. 2.17). It is important to remember that the nidus is the lesion that must be ablated for effective treatment and that the sclerosis, which may regress after treatment, is reactive to the nidus.
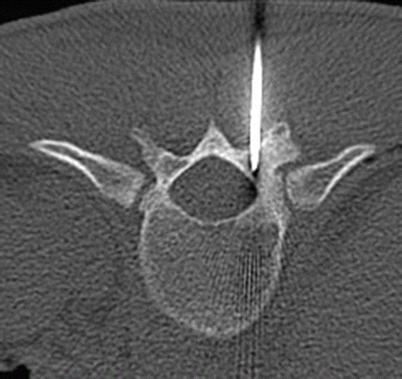

Fig. 2.17
Axial CT of T11 shows CT-guided percutaneous placement of a probe within the nidus in the T11 lamina for radiofrequency ablation
Osteoblastoma
Osteoblastoma is a benign lesion representing approximately 1 % of all primary bone tumors. The histology is very similar to that of osteoid osteoma but the size, radiographic appearance, and distribution of these lesions differ. Most patients present between the ages of 10 and 30 years, with a male to female ratio of 2:1. Pain is the most common symptom but is less severe that that caused by osteoid osteoma. With osteoblastoma, the pain is not more severe at night and is not as frequently relieved by NSAIDs. There is a predilection for the spine which is the site of approximately 30 % of lesions. Lesions are distributed equally among all segments but favor the posterior elements with common extension into the vertebral body. Lesions in the long bones most often involve the femur and tibia, occurring most commonly in the metaphysis or distal diaphysis. Whereas osteoid osteomas are stable and can show spontaneous regression, osteoblastomas continue to grow slowly. Osteoblastomas can have three patterns. The first pattern appears similar to a giant osteoid osteoma but measures greater than 1.5–2 cm and has less reactive sclerosis, although periostitis has been noted to be more prominent. The second pattern is that of an expansile lytic lesion appearing similar to an aneurysmal bone cyst but also sometimes containing punctate areas of mineralization (Fig. 2.18). This is the most common appearance of lesions in the spine. The third pattern is an aggressive-appearing lesion with bony destruction and scattered mineralization or soft tissue mass. Up to 20 % of lesions have associated aneurysmal bone cyst.
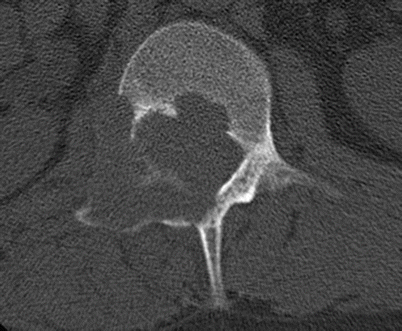

Fig. 2.18
Osteoblastoma in a 30-year-old man. Axial CT shows an expansile lytic lesion in the posterior elements of L1 on the right with cortical disruption. The differential diagnosis was osteoblastoma or aneurysmal bone cyst based on the location. The diagnosis of osteoblastoma was made with biopsy
CT is the best imaging modality for the diagnosis of osteoblastoma of the spine. MRI is nonspecific and will show low to intermediate T1 signal and variable T2 signal with foci that demonstrate low signal intensity corresponding to calcification. Bone scintigraphy will show marked increased Tc99 MDP uptake. The differential diagnosis for an expansile lesion in the posterior elements of the spine is an aneurysmal bone cyst. Treatment is radical surgical excision, and the recurrence rate is 10–15 %.
Osteosarcoma
Osteosarcoma is the most common nonhematologic primary malignancy of the bone, accounting for approximately 35 % of primary bone tumors. The tumor is characterized by sarcomatous stroma that has the ability to produce osteoid or immature bone. Although there can be a predominance of other tissue types such as chondroid or fibrous tissue, even a small amount of osteoid production designates the tumor as an osteogenic sarcoma. Osteosarcoma has a bimodal age distribution, with most cases occurring in childhood and a smaller second peak in older adults. There are varying histological subtypes, the majority of which can be predicted on the basis of their radiographic appearance, location, and patient age. In addition to primary osteosarcoma, which can be intramedullary or a surface lesion, osteosarcoma can be secondary, occurring with Paget’s, prior radiation treatment, bone infarct, or other benign lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








