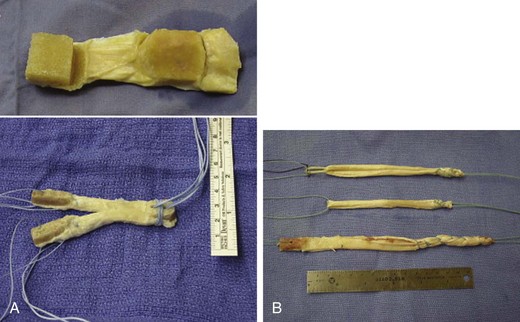
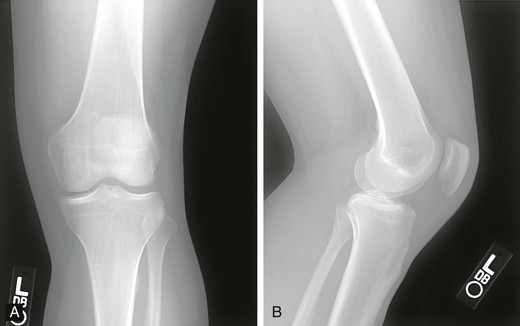
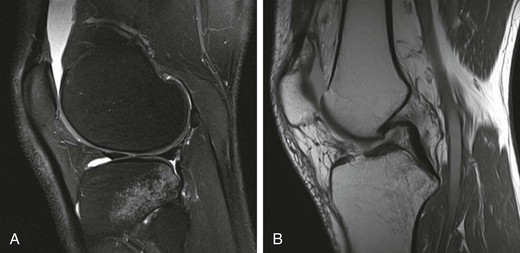
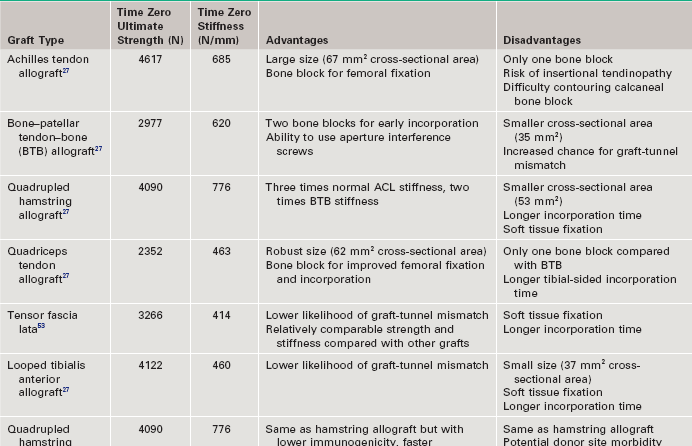
Chapter 73 The use of allografts has enjoyed considerable clinical success in many soft tissue reconstructive procedures, including anterior cruciate ligament (ACL) reconstruction, one of the most common orthopedic procedures performed today. With more than 250,000 new ACL ruptures reportedly each year,12,13 estimates indicate that 300,000 ACL reconstructions are performed annually, with approximately 240,000 (80%) performed with autograft tissue and 60,000 (20%) with allograft tissue.7 Rationales for increased use of allograft tissue include more effective sterilization procedures,14 organized collection and distribution of tissue, and increased confidence in the overall stability of allografts.15–17 The orthopedic surgeon has a number of graft options for reconstructive surgery of the ACL. Bone–patellar tendon–bone (BPTB) and hamstring autografts have historically been used for ACL reconstructive surgery, with numerous peer-reviewed studies reporting favorable clinical outcomes.18–21 However, the use of allograft tissue for ACL reconstruction has increased.18–20 These grafts avoid potential donor site morbidity associated with hamstring or patellar tendon graft harvest, including donor site pain,1,2 patellar fracture,3 patellar tendon rupture,4,5 saphenous nerve injury,6 and residual extensor mechanism or hamstring weakness.1,22–24 Currently available allograft tissue includes BPTB; Achilles, hamstring, or tibialis tendon; and tensor fascia lata (Fig. 73-1). The increasing demand for allograft tissue in ACL reconstruction is attributed to potentially decreased donor site morbidity and postoperative pain, decreased operating room time, increasing availability from tissue banks, and improved cosmesis. However, potential disadvantages include cost,25,26 availability, reliability of vendors, slower graft incorporation, the possibility of disease transmission and/or immunologic response,7 and a potentially increased risk of failure among younger athletes.8 Ideal ACL grafts have the following characteristics27: • Structural properties similar to native ACL • Should allow secure fixation • Should permit rapid biologic incorporation and ligamentization Contributing Mechanisms to Anterior Cruciate Ligament Injuries • A majority of injuries (70%) are noncontact, involving deceleration and a dynamic valgus or adduction moment, with the quadriceps contracted against an extended knee. • A minority of injuries (30%) are contact, with fixed lower leg and internal rotation, valgus stress, or hyperextension injury. • The classic description is of a popping sensation followed by immediate pain and swelling of the knee. • The patient reports a feeling of instability or giving-way episodes with cutting or pivoting activity. • Assessment of standing alignment is critical for every patient. Significant varus alignment increases the risk of failure after ACL reconstruction and may warrant realignment with associated osteotomy. • Gait should be examined to assess for associated varus thrust. The presence of a thrust often indicates associated lateral collateral and posterolateral (PL) corner complex insufficiency, and a failure to recognize this will increase the risk of failure of the ACL reconstruction. • Effusion and hemarthrosis may be present. • Preexisting scars may provide insight into previous surgical procedures and/or graft source. • Range of motion. It is critical to achieve resolution of effusion and restoration of motion before ACL reconstructive surgery to minimize risk of postoperative stiffness and arthrofibrosis. A mechanical block to full extension or flexion despite resolution of an effusion is often indicative of an associated bucket-handle meniscal tear.28 • Joint line tenderness is often indicative of an associated meniscal injury, or secondary to the typical bone contusion pattern of a pivot-shift event. • All tests should be performed on the involved and the contralateral limbs, because side-to-side differences are most important in the detection of subtle differences in stability and ligamentous integrity. • Anterior drawer and Lachman tests are critical for assessment of anterior translational instability. Relative differences between limbs are more important than absolute measurements of translation to indicate ACL insufficiency. • Anteromedial (AM) rotatory stability to assess the integrity of the medial collateral and posterior oblique ligaments. Occult posteromedial corner injury can increase the risk of ACL reconstruction failure.29–31 • Anterolateral rotatory instability and “dial” testing to assess integrity of the lateral collateral and PL corner complex. Missed PL corner injury is a well-recognized cause of failure after ACL reconstruction.29–31 • Varus and valgus examination of the knee in full extension and 30 degrees of flexion to assess collateral ligament integrity. Associated collateral ligament injury may warrant delayed surgical intervention or operative repair and must be addressed with ACL injury to avoid failure of the reconstruction. • Pivot-shift testing with axial load and valgus stress is critical to assess functional instability and rotational instability secondary to ACL insufficiency. Note that the pivot-shift test result may be negative in the setting of an associated bucket-handle tear of the meniscus or medial collateral ligament (MCL) insufficiency that prevents valgus loading of the knee. Presence of fat lobules in aspirated fluid may be confirmatory of associated fracture or osteochondral trauma. • Standing, long-leg radiographs are essential for assessment of mechanical alignment of the extremities. Asymmetric varus alignment must be addressed to avoid failure of the ACL reconstruction. • Anteroposterior, lateral, and sunrise views of the knee should be obtained. Loose bodies, spine avulsion fractures, and occult plateau fractures may be identified. Segond fractures with lateral capsular avulsion fragments are pathognomonic of ACL injury but are not universally present with ACL injury. The presence of patella alta, patella baja, or tibial tubercle apophysitis may be a relative contraindication to autogenous patellar tendon graft selection. • A flexion weight-bearing (Rosenberg) view should be obtained to assess for significant tibiofemoral joint space narrowing. The presence of significant osteoarthritic changes may be a relative contraindication to ACL reconstruction. Magnetic resonance imaging has a high sensitivity (94.4%) and specificity (94.3%) for full-thickness tear9 (Fig. 73-3). Indirect Signs of Anterior Cruciate Ligament Tear32: The approach to graft election for ACL reconstruction must be determined on an individual basis after a thorough review of the risks and benefits of autograft and allograft sources. Patients should make an informed decision about graft type used for ACL reconstruction. However, there are populations of patients in whom allograft may be preferable over autograft7,9: • Patients 40 years of age and older may have an equivalent rate of failure with autograft or allograft ACL reconstruction.8 • Patients with decreased functional demands or expectations, including a return to low-intensity, recreational sports • Skeletally immature athletes, to avoid physeal or apophyseal injury associated with autogenous graft harvest. • Patients with history of patellofemoral pain or previous patellar surgery (realignment, tendon repair, fracture). • Laborers or athletes who frequently kneel, such as roofers, masons, firefighters, wrestlers, baseball catchers, and football linemen. • Patients undergoing revision ACL or multiligament reconstructive surgery. Patients in whom allograft is less desirable include the following: The surgeon has multiple options when it comes to choosing allograft tissue, including patellar tendon, hamstring, Achilles tendon, tibialis anterior tendon, and tensor fascia lata. These may be procured from one of the many tissue banks currently in practice. It has been reported that there are more than 80 tissue banks that are current members of the AATB, with at least 60 more banks that are not currently members.22 It is prudent for the surgeon to be knowledgeable about the soft tissue bank’s methods of procurement, sterilization, and preparation before using its allografts, because these factors can significantly affect a patient’s outcome. Although the number of infections is likely underreported, allograft transplantation infection rates are very low; the reported incidence is less than 1% (0.0004% to 0.014%).33,34 Despite this low incidence, many surgeons continue to be concerned with the risk of allograft disease transmission.33 This phenomenon has been well documented35,36 with regard to transmission of human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), group A streptococcus, Clostridium species, and prions. In addition, many surgeons have concerns about regulatory practices and the biomechanical properties of tissue after sterilization. Currently, all human cells or tissues intended for transplantation into a human recipient are regulated as HCT/P, or “human cell, tissue, and cellular and tissue-based product.”34,37 Any institution that recovers, processes, stores, or handles allograft tissue consequently not only must register with the Center for Biologics Evaluation and Research33 of the U.S. Food and Drug Administration (FDA), but also is strongly encouraged to seek certification from the AATB’s voluntary accreditation program, which ensures that tissue banks that seek its certification follow strict guidelines for tissue processing. Because of past disease transmission, the FDA now mandates that all donor tissue be screened for HIV types 1 and 2, HBV, HCV, Treponema pallidum, and human transmissible spongiform encephalopathies with use of advanced nucleic acid testing (NAT) techniques.34 NAT sharply reduces the diagnostic window and excludes donors with high viremia.38 In addition, the AATB requires members to conduct NAT testing and provide negative Clostridium and Streptococcus pyogenes tissue culture results.34,37 Although accreditation by the AATB is not required, tissue banks are urged to seek certification. Lack of accreditation can be a warning to the surgeon about the quality of the allografts provided by the tissue bank. After appropriate donor screening, including exhaustive reviews of medical and social histories for risk factors for infectious disease, allograft tissue must undergo processing to ensure that pathogens are not concomitantly transferred. It should be noted that the Current Good Tissue Practice does not necessarily mandate aseptic handling or sterilization before transplantation of donor tissue; however, tissue recovery is typically performed with use of aseptic technique, in a standard operating room setting.34,37 Tissue procured under these conditions is not necessarily sterile—contamination may still be introduced by health care workers or the donor’s endogenous flora.34 After procurement, specimens are sterilized. There is currently no single preferred sterilization process; however, the two main processes for sterilization are irradiation and proprietary chemical processing. The FDA maintains that biologic medical devices are acceptable if a sterility assurance level (SAL) of 10−3 (1 in 1000 chance that a living microbe exists) is attained. Human tissues may be considered sterile only if these specimens have an SAL of 10−6. However, allografts that have not been treated with gamma or electron-beam irradiation or ethylene oxide are not likely to be sterile.39 Irradiation of soft tissue allografts is a delicate balancing act. Gamma radiation sterilization is an effective technique33,40 that works by inducing excitation of molecules and ions for radical-induced chemical reactions, which subsequently leads to demise of pathogens. Although irradiation of 25 to 40 kGy can inactivate HIV and eliminate spores, the free-radical formation resulting from these doses has been shown to significantly alter the biomechanics of soft tissue grafts. Generation of free radicals distorts the integrity of the allograft tissue, which has been demonstrated in a number of studies showing decreasing allograft biomechanical integrity and enzyme resistance in irradiated tendons41,42 with increasing doses of irradiation (Table 73-1).43–45 The underlying reasons for this poor biomechanical integrity are not yet well understood. Gamma irradiation above 20 to 25 kGy is now seldom used because of this concern.22
Allografts for Anterior Cruciate Ligament Reconstruction
Preoperative Considerations
History
Typical History
Physical Examination
Specific Tests
Imaging
Magnetic Resonance Imaging
Indications and Contraindications
Surgical Technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Allografts for Anterior Cruciate Ligament Reconstruction