Chapter 7 Ablation Energy Sources
Radiofrequency Ablation
Biophysics of Radiofrequency Energy
Radiofrequency (RF) refers to the portion of the electromagnetic spectrum in which electromagnetic waves can be generated by alternating current fed to an antenna.1 Electrosurgery ablation currently uses hectomeric wavelengths found in band 6 (300 to 3000 kHz), which are similar to those used for broadcast radio. However, the RF energy is electrically conducted, not radiated, during catheter ablation. The RF current is similar to low-frequency alternating current or direct current with regard to its ability to heat tissue and create a lesion, but it oscillates so rapidly that cardiac and skeletal muscles are not stimulated, thereby avoiding induction of arrhythmias and decreasing the pain perceived by the patient. RF current rarely induces rapid polymorphic arrhythmias; such arrhythmias can be observed in response to low-frequency (60-Hz) stimulation. Frequencies higher than 1000 kHz are also effective in generating tissue heating; however, such high frequencies are associated with considerable energy loss along the transmission line. Therefore, frequencies of the RF current commonly used are in the range of 300 to 1000 kHz, a range that combines efficacy and safety.2,3
Radiofrequency Energy Delivery
Delivery of RF energy depends on the establishment of an electrical circuit involving the human body as one of its in-series elements. The RF current is applied to the tissue via a metal electrode at the tip of the ablation catheter and is generally delivered in a unipolar fashion between the tip electrode and a large dispersive electrode (indifferent electrode, or ground pad) applied to the patient’s skin. The polarity of connections from the electrodes to the generator is not important because the RF current is an alternating current. Bipolar RF systems also exist, in which the current flows between two closely apposed small electrodes, thus limiting the current flow to small tissue volumes interposed between the metal conductors. Bipolar systems, partly because of their relative safety, are now the preferred tools in electrosurgery (oncology, plastic surgery, and ophthalmology). Their clinical application for catheter-based ablation has not yet been evaluated.2,3
The system impedance comprises the impedance of the generator, transmission lines, catheter, electrode-tissue interface, dispersive electrode-skin interface, and interposed tissues. As electricity flows through a circuit, every point of that circuit represents a drop in voltage, and some energy is dissipated as heat. The point of greatest drop in line voltage represents the area of highest impedance and is where most of that electrical energy becomes dissipated as heat. Therefore, with excessive electrical resistance in the transmission line, the line actually warms up and power is lost. Current electrical conductors from the generator all the way through to the patient and from the dispersive electrode back to the generator have low impedance, to minimize power loss.2,3
With normal electrode-tissue contact, only a fraction of all power is effectively applied to the tissue. The rest is dissipated in the blood pool and elsewhere in the patient. With an ablation electrode in contact with the endocardial wall, part of the electrode contacts tissue and the rest contacts blood, and the RF current flows through both the myocardium and the blood pool in contact with the electrode. The distribution between both depends on the impedance of both routes and also on how much electrode surface contacts blood versus endocardial wall. Whereas tissue heating is the target of power delivery, the blood pool is the most attractive route for RF current because blood is a better conductor and has significantly lower impedance than tissue and because the contact between electrode and blood is often better than with tissue. Therefore, with normal electrode-tissue contact, much more power is generally delivered to blood than to cardiac tissue.2,4
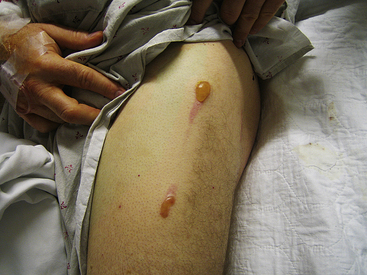
After leaving the electrode-blood-tissue interface, the current flows through the thorax to the indifferent electrode. Part of the RF power is lost in the patient’s body, including the area near the patch. Dissipation of energy can occur at the dispersive electrode site (at the contact point between that ground pad and the skin) to a degree that can limit lesion formation. In fact, if ablation is performed with a high-amplitude current (more than 50 W) and skin contact by the dispersive electrode is poor, it is possible to cause skin burns (Fig. 7-1).1 Nevertheless, because the surface area of the ablation electrode (approximately 12 mm2) is much smaller than that of the dispersive electrode (approximately 100 to 250 cm2), the current density is higher at the ablation site, and heating occurs preferentially at that site, with no significant heating occurring at the dispersive electrode.5
The dispersive electrode may be placed on any convenient skin surface. The geometry of the RF current field is defined by the geometry of the ablation electrode and is relatively uniform in the region of volume heating. Thus, the position of the dispersive electrode (on the patient’s back or thigh) has little effect on impedance, voltage, current delivery, catheter tip temperature, or geometry of the resulting lesion.2,3,5
The size of the dispersive electrode, however, is important. Sometimes it is advantageous to increase the surface area of the dispersive electrode. This increase leads to lower impedance, higher current delivery, increased catheter tip temperatures, and more effective tissue heating. This is especially true in patients with baseline system impedance greater than 100 Ω. Moreover, when the system is power limited, as with a 50-W generator, heat production at the catheter tip varies with the proportion of the local electrode-tissue interface impedance to the overall system impedance. If the impedance at the skin-dispersive electrode interface is high, then a smaller amount of energy is available for tissue heating at the electrode tip. Therefore, when ablating certain sites, adding a second dispersive electrode or optimizing the contact between the dispersive electrode and skin should result in relatively more power delivery to the target tissue.5,6
Tissue Heating
During alternating current flow, charged carriers in tissue (ions) attempt to follow the changes in the direction of the alternating current, thus converting electromagnetic (current) energy into molecular mechanical energy or heat. This type of electric current-mediated heating is known as ohmic (resistive) heating. Using Ohm’s law, with resistive heating, the amount of power (= heat) per unit volume equals the square of current density times the specific impedance of the tissue. With a spherical electrode, the current flows outward radially, and current density therefore decreases with the square of distance from the center of the electrode. Consequently, power dissipation per unit volume decreases with the fourth power of distance. The thickness of the electrode eliminates the first steepest part of this curve, however, and the decrease in dissipated power with distance is therefore somewhat less dramatic.2,3
Approximately 90% of all power that is delivered to the tissue is absorbed within the first 1 to 1.5 mm from the electrode surface. Therefore, only a thin rim of tissue in immediate contact with the RF electrode is directly heated (within the first 2 mm of depth from the electrode). The remainder of tissue heating occurs as a result of heat conduction from this rim to the surrounding tissues. On initiation of fixed-level energy application, the temperature at the electrode-tissue interface rises monoexponentially to reach steady state within 7 to 10 seconds, and the steady state is usually maintained between 80° and 90°C. However, whereas resistive heating starts immediately with the delivery of RF current, conduction of heat to deeper tissue sites is relatively slow and requires 1 to 2 minutes to equilibrate (thermal equilibrium). Therefore, the rate of tissue temperature rise beyond the immediate vicinity of the RF electrode is much slower, resulting in a steep radial temperature gradient as tissue temperature decreases radially in proportion to the distance from the ablation electrode; however, deep tissue temperatures continue to rise for several seconds after interruption of RF delivery (the so-called thermal latency phenomenon).4 Therefore, RF ablation requires at least 30 to 60 seconds to create full-grown lesions. In addition, when temperature differences between adjacent areas develop because of differences in local current density or local heat capacity, heat conducts from hotter to colder areas, thus causing the temperature of the former to decrease and that of the latter to increase. Furthermore, heat loss to the blood pool at the surface and to intramyocardial vessels determines the temperature profile within the tissue.2,3
At steady state, the lesion size is proportional to the temperature measured at the interface between the tissue and the electrode, as well as to the RF power amplitude. By using higher powers and achieving higher tissue temperatures, the lesion size can be increased. However, once the peak tissue temperature exceeds the threshold of 100°C, boiling of the plasma at the electrode-tissue interface can ensue. When boiling occurs, denatured serum proteins and charred tissue form a thin film that adheres to the electrode, thus producing an electrically insulating coagulum, which is accompanied by a sudden increase in electrical impedance that prevents further current flow into the tissue and further heating.2,3
Convective Cooling
The dominant factor opposing effective heating of myocardium is the convective heat loss into the circulating blood pool. Because the tissue surface is cooled by the blood flow, the highest temperature during RF delivery occurs slightly below the endocardial surface. Consequently, the width of the endocardial lesion matures earlier than the intramural lesion width (20 seconds versus 90 to 120 seconds). Therefore, the maximum lesion width is usually located intramurally, and the resultant lesion is usually teardrop shaped, with less necrosis of the superficial tissue.3
As the magnitude of convective cooling increases (e.g., unstable catheter position, poor catheter-tissue contact, or high blood flow in the region of catheter position), there is decreased efficiency of heating as more energy is carried away in the blood and less energy is delivered to the tissue. When RF power is limited, lesion size is reduced by such convective heat loss. On the other hand, when RF power delivery is not limited, convective cooling allows for more power to be delivered into the tissue, and higher tissue temperatures can be achieved (despite low temperatures measured by the catheter tip sensors), resulting in larger lesion size, without the risk of overheating and coagulum formation.2,3
The concept of convective cooling can explain why there are few coronary complications with conventional RF ablation. Coronary arteries act as a heat sink; substantive heating of vascular endothelium is prevented by heat dissipation in the high-velocity coronary blood flow, even when the catheter is positioned close to the vessel. Although this is advantageous, because coronary arteries are being protected, it can limit success of the ablation lesion if a large perforating artery is close to the ablation target.5
Catheter Tip Temperature
Ablation catheter tip temperature depends on tissue temperature, convective cooling by the surrounding blood, tissue contact of the ablation electrode, electrode material and its heat capacity, and type and location of the temperature sensor.2,7
Catheter tip temperature is measured by a sensor located in the ablation electrode. There are two different types of temperature sensors: thermistors and thermocouples. Thermistors require a driving current, and the electrical resistance changes as the temperature of the electrical conductor changes. More frequently used are thermocouples, which consist of copper and constantan wires and are incorporated in the center of the ablation electrode. Thermocouples are based on the so-called Seebeck effect; when two different metals are connected (sensing junction), a voltage can be measured at the reference junction that is proportional to the temperature difference between the two metals.2,7
Several other factors can increase the disparity between catheter tip temperature and tissue temperature, including catheter tip irrigation, large ablation electrode size, and poor electrode-tissue contact. Catheter tip irrigation increases the disparity between tissue temperature and electrode temperature because it results in cooling of the ablation electrode, but not the tissue. With a large electrode tip, a larger area of the electrode tip is exposed to the cooling effects of the blood flow than with standard tip lengths, thus resulting in lower electrode temperatures. Similarly, with poor electrode-tissue contact, less electrode material is in contact with the tissue, and heating of the tip by the tissue occurs at a lower rate, resulting in relatively low tip temperatures.3,6–8
Pathophysiology of Lesion Formation by Radiofrequency Ablation
Cellular Effects of Radiofrequency Ablation
The primary mechanism of tissue injury by RF ablation is likely to be thermally mediated. Hyperthermic injury to the myocyte is both time- and temperature-dependent, and it can be caused by changes in the cell membrane, protein inactivation, cytoskeletal disruption, nuclear degeneration, or other potential mechanisms.2,3
Experimentally, the resting membrane depolarization is related to temperature. In the low hyperthermic range (37° to 45°C), little tissue injury occurs, and a minor change may be observed in the resting membrane potential and action potential amplitude. However, action potential duration shortens significantly, and conduction velocity becomes greater than at baseline. In the intermediate hyperthermic range (45° to 50°C), progressive depolarization of the resting membrane potential occurs, and action potential amplitude decreases. Additionally, abnormal automaticity is observed, reversible loss of excitability occurs, and conduction velocity progressively decreases. In the high temperature ranges (higher than 50°C), marked depolarization of the resting membrane potential occurs, and permanent loss of excitability is observed. Temporary (at temperatures of 49.5° to 51.5°C) and then permanent (at 51.7° to 54.4°C) conduction block develops, and fairly reliable irreversible myocardial injury occurs with a short hyperthermic exposure.3
RF ablation typically results in high temperatures (70° to 90°C) for a short time (up to 60 seconds) at the electrode-tissue interface, but significantly lower temperatures at deeper tissue sites. This leads to rapid tissue injury within the immediate vicinity of the RF electrode but relatively delayed myocardial injury with increasing distance from the RF electrode. Therefore, although irreversible loss of EP function can usually be demonstrated immediately after successful RF ablation, this finding can be delayed because tissue temperatures continue to rise somewhat after termination of RF energy delivery (thermal latency phenomenon). This effect can account for the observation that patients undergoing AV node (AVN) modification procedures who demonstrate transient heart block during RF energy delivery can progress to persisting complete heart block, even if RF energy delivery is terminated immediately. Reversible loss of conduction can be demonstrated within seconds of initiating the RF application, which can be caused by an acute electrotonic effect. On the other hand, there can be late recovery of electrophysiological (EP) function after an initial successful ablation.5
Tissue Effects of Radiofrequency Ablation
Changes in myocardial tissue are apparent immediately on completion of the RF lesion. Pallor of the central zone of the lesion is attributable to denaturation of myocyte proteins (principally myoglobin) and subsequent loss of the red pigmentation. Slight deformation, indicating volume loss, occurs at the point of catheter contact in the central region of lesion formation. The endocardial surface is usually covered with a thin fibrin layer and, occasionally, if a temperature of 100°C has been exceeded, with char and thrombus (Fig. 7-2). In addition, a coagulum (an accumulation of fibrin, platelets, and other blood and tissue components) can form at the ablation electrode because of the boiling of blood and tissue serum.2
On sectioning, the central portion of the RF ablation lesion shows desiccation, with a surrounding region of hemorrhagic tissue and then normal-appearing tissue. Histologic examination of an acute lesion shows typical coagulation necrosis with basophilic stippling consistent with intracellular calcium overload. Immediately surrounding the central lesion is a region of hemorrhage and acute monocellular and neutrophilic inflammation. The progressive changes seen in the evolution of an RF lesion are typical of healing after any acute injury. Within 2 months of the ablation, the lesion shows fibrosis, granulation tissue, chronic inflammatory infiltrates, and significant volume contraction. The lesion border is well demarcated from the surrounding viable myocardium without evidence of a transitional zone. This likely accounts for the absence of proarrhythmic side effects of RF catheter ablation. As noted, because of the high-velocity blood flow within the epicardial coronary arteries, these vessels are continuously cooled and are typically spared from injury, despite nearby delivery of RF energy. However, high RF power delivery in small hearts, such as in pediatric patients, or in direct contact with the vessel can potentially cause coronary arterial injury.3
The border zone around the acute pathological RF lesion accounts for several phenomena observed clinically. The border zone is characterized by marked ultrastructural abnormalities of the microvasculature and myocytes acutely, as well as a typical inflammatory response later. The most thermally sensitive structures appear to be the plasma membrane and gap junctions, which show morphological changes as far as 6 mm from the edge of the pathological lesion. The border zone accounts for documented effects of RF lesion formation well beyond the acute pathological lesion. The progression of the EP effects after completion of the ablation procedure can be caused by further inflammatory injury and necrosis in the border zone region that result in late progression of physiological block and a delayed cure in some cases. On the other hand, initial stunning and then early or late recovery of function can be demonstrated in the border zone, thus accounting for the recovery of EP function after successful catheter ablation in the clinical setting, which can be caused by healing of the damaged, but surviving, myocardium.2
Determinants of Lesion Size
Lesion size is defined as the total volume or dimensions (width and depth) of the lesion. The size of the lesion created by RF power is determined by the amount of tissue heated to more than the critical temperature for producing irreversible myocardial damage (50°C). As noted, only a thin rim (1 to 2 mm) of tissue immediately under the ablating electrode is directly heated. This heat then radiates to adjacent tissue; however, conduction of heat to deeper tissue sites is relatively slow and very inefficient. The distance at which temperature drops to less than 50°C delimits the depth of lesion formation. The use of higher-power output to achieve higher tissue temperatures results in larger lesions by raising the temperature of the rim of resistively heated tissue to substantially more than 50°C for deeper tissue to reach the 50°C threshold required for tissue necrosis. However, the rim of heated tissue in direct contact with the ablating electrode conducts not only to deeper tissue but also to the electrode tip itself. Higher electrode temperatures either limit further energy delivery (in temperature-controlled power delivery mode) or increase electrode impedance as a result of coagulum formation, or both; these effects potentially limit lesion size. Furthermore, tissue temperatures higher than 100°C are unsafe because they are associated with higher risk of steam “pops.” Cooling of the ablating electrode (passively by using a larger electrode length or actively by using catheter irrigation) can help diminish electrode heating, allowing for greater power delivery and creation of larger lesions.9
Electrode-Tissue Contact
The efficiency of energy transfer to the myocardium largely depends on electrode-tissue contact. An improvement in tissue contact leads to a higher amount of RF power that can be effectively delivered to the tissue. Consequently, the same tissue temperatures and lesion size can be reached at a much lower power level. However, at a certain moderate contact force, further increase in contact firmness results in progressively smaller lesions because a lesser amount of RF power is required to reach target temperature.4
Additionally, the surface area at the electrode-tissue interface influences the lesion size. When the catheter is wedged (i.e., between ventricular trabeculae or under a valvular leaflet), the electrode surface area exposed to tissue can be much higher. With half the electrode in contact with blood and the other half in contact with (twice) more resistive tissue, the amount of power delivered to blood is two times higher than the amount of power delivered to tissue (as compared with six times with 25% contact). The result is a more than twofold increase in tissue heating. In practice, however, power output rarely reaches 50 W in these situations; a temperature rise of the ablation electrode signals excessive tissue heating and limits power delivery.4
Electrode Orientation
When the catheter tip is perpendicular to the tissue surface, a much smaller surface area is in contact with the tissue (versus that exposed to the cooling effect of blood flow) than when the catheter electrode tip is lying on its side. Clinically, perpendicular electrode orientation yields larger lesion volumes and uses less power than parallel electrode orientation. However, if the RF power level is adjusted to maintain a constant current density, lesion size will increase proportionally to the electrode-tissue contact area, which is larger with parallel tip orientation. Lesion depth is only slightly affected by catheter tip orientation using 4-mm-long tip catheters, but lesions are slightly longer in the parallel orientation as compared with the perpendicular orientation. Moreover, the character of the lesion created with temperature control depends on the placement of the temperature sensors relative to the portion of the electrode in contact with tissue. Thus, the orientation of the electrode and of its temperature sensors determines the appropriate target temperature required to create maximal lesions while avoiding coagulum formation caused by overheating at any location within the electrode-tissue interface.2,3,10
Electrode Length
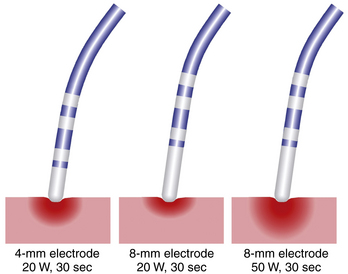
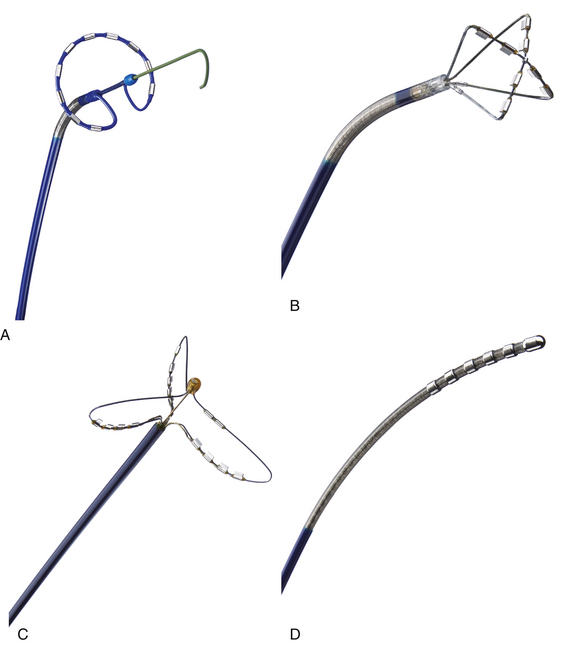
Ablation catheters have tip electrodes that are conventionally 4 mm long and are available in sizes up to 10 mm long (see Fig. 4-6). An increased electrode size reduces the interface impedance with blood and tissue, but the impedance through the rest of the patient remains the same. The ratio between interface impedance and the impedance through the rest of the patient is thus lower with an 8-mm electrode than with a 4-mm electrode, which reduces the efficiency of power transfer to the tissue. Therefore, with the same total power, lesions created with a larger electrode are always smaller than lesions created with a smaller electrode (Fig. 7-3). A larger electrode size also creates a greater variability in power transfer to the tissue because of greater variability of tissue contact, and tissue contact becomes much more dependent on catheter orientation with longer electrodes. Consequently, an 8-mm electrode may require a 1.5 to 4 times higher power level than a 4-mm electrode to create the same lesion size.4
When the power is not limited, however, catheters with large distal electrodes create larger lesions, both by increasing the ablation electrode surface area in contact with the bloodstream, thus resulting in an augmented convective cooling effect, and by increasing the volume of tissue directly heated because of an increased surface area at the electrode-tissue interface (see Fig. 7-3). However, this assumes that the electrode-tissue contact, tissue heat dissipation, and blood flow are uniform throughout the electrode-tissue interface. As the electrode size increases, the likelihood that these assumptions are true diminishes because of variability in cardiac chamber trabeculations and curvature, tissue perfusion, and intracardiac blood flow, which affect the heat dissipation and tissue contact. These factors result in unpredictable lesion size and uniformity for electrodes more than 8 mm long.2,3
There is a potential safety concern with the use of long ablation electrodes because of nonuniform heating, with maximal heating occurring at the electrode edges. Thus, large electrode-tipped catheters with only a single thermistor can underestimate maximal temperature and allow char formation and potential thromboembolic complications. Catheter tips with multiple temperature sensors at the electrode edges may be preferable for temperature feedback. In addition, the greater variation in power delivered to the tissue and the greater discrepancy between electrode and tissue temperature make it difficult to avoid intramural gas explosions and blood clot formation. Another point of concern is that the formation of blood clots may only minimally affect electrode impedance by covering a much smaller part of the electrode surface. Therefore, the lower electrode temperature and the absence of any impedance rise may erroneously suggest a safer ablation process.4
The principal limitations of a large ablation electrode (8 to 10 mm in length) are the reduction in mobility and the flexibility of the catheter, which can impair positioning of the ablation electrode, and a reduction in the resolution of recordings from the ablation electrode, thus making it more difficult to identify the optimal ablation site. A larger electrode dampens the local electrogram, especially that of the distal electrode. With an 8- or 10-mm long distal and a 1-mm short proximal ring electrode, the latter can be the main source for the bipolar electrogram; this then confuses localization of the optimal ablation site. In contrast, a smaller electrode improves mapping accuracy and feedback of tissue heating. Its only drawback is the limited power level that can be applied to the tissue.4
Ablation Electrode Material
Although platinum-iridium electrodes have been the standard for most RF ablation catheters, gold exhibits excellent electrical conductive properties, as well as a more than four times greater thermal conductivity than platinum (300 versus 70 W/m °K), although both materials have similar heat capacities (130 and 135 J/kg °K). The higher thermal conductivity of gold can potentially lead to a higher mean rate of power because of better heat conduction at the tissue-electrode interface and to enhanced cooling as a result of heat loss to the surrounding blood with this electrode material.11 Therefore, gold electrodes allow for greater power delivery to create deeper lesions at a given electrode temperature without impedance increases.12 Enhanced electrode cooling allows for more RF power to be applied at constant temperature, before the temperature limit is reached or before the impedance of the electrode rises.4 However, the higher thermal conductivity of gold electrodes is no longer an advantage in areas of low blood flow (e.g., among myocardial trabeculae), where convective cooling at the electrode tip is minimal. Under these circumstances, electrode materials with a low thermal conductivity can produce larger lesions.3,11
Conflicting results were observed in clinical studies comparing 8-mm gold-tip with platinum-iridium–tip catheters for ablation of the cavotricuspid isthmus.13 During catheter ablation of the slow AVN pathway in patients with AVN reentrant tachycardia (AVNRT), no significant differences were observed between 4-mm gold-tip and platinum-iridium–tip catheters in the primary endpoint or in the increases of power or temperature at any of the measured time points. However, ablation with gold electrodes seemed to be safe and well tolerated and specifically did not increase the risk of AV block. Interestingly, a significant reduction of charring on gold tips was observed, compared with platinum-iridium material, a finding suggesting a possible advantage of this material beyond its better conduction properties.11
Reference Patch Electrode Location And Size
The RF current path and skin reference electrode interface present significant impedance for the ablation current flow, thereby dissipating part of the power. Increasing patch size (or using two patches) provides for increased heating at the electrode-endocardium interface and thus increases ablation efficiency and increases lesion size. On the other hand, the position of the dispersive electrode (on the patient’s back or thigh) has little effect on the size of the resulting lesion.5
Blood Flow
The ablation electrode temperature is dependent on the opposing effects of heating from the tissue and cooling by the blood flowing around the electrode. Because lesion size is primarily dependent on the RF power delivered to the tissue, lesion size varies with the magnitude of local blood flow. At any given electrode temperature, the RF power delivered to the tissue is significantly reduced in areas of low local blood flow (e.g., deep pouch in the cavotricuspid isthmus, dilated and poorly contracting atria, and dilated and poorly contracting ventricles). The reduced cooling associated with low blood flow causes the electrode to reach the target temperature at lower power levels, and if the ablation lesion is temperature-controlled, power delivery will be limited. In these locations, increasing electrode temperature to 65° or 70°C only minimally increases RF power and increases the risk of thrombus formation and impedance rise. In contrast, increasing local blood flow is associated with increased convective cooling of the ablation electrode. Consequently, more power is delivered to the tissue to reach and maintain target temperature, thus resulting in larger lesion volumes.2,3,7
Radiofrequency System Polarity
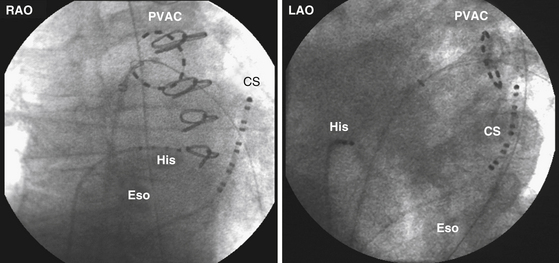
Most RF lesions are created by applying energy in a unipolar fashion between an ablating electrode touching the myocardium and a grounded reference patch electrode placed externally on the skin. The unipolar configuration creates a highly localized lesion, with the least amount of surface injury. Energy can also be applied in a bipolar mode between two endocardial electrodes. Bipolar energy delivery produces larger lesions than unipolar delivery. A novel ablation system (GENius, Ablation Frontiers, Carlsbad, Calif.) has been developed for ablation of atrial fibrillation (AF), in which duty-cycled and alternating unipolar and bipolar (between adjacent electrodes) energy RF ablation is used to create contiguous lesions in the pulmonary vein (PV) antrum using a ring catheter with 10 electrodes (Pulmonary Vein Ablation Catheter [PVAC], Ablation Frontiers, Medtronic Inc., Minneapolis, Minn.) (Figs. 7-4 and 7-5).14 Other catheter designs, which use the same multichannel duty-cycled RF generator, also have been developed for left atrial ablation of fractionated electrograms (Multi-Array Ablation Catheter [MAAC], Ablation Frontiers), septal ablation (Multi-Array Septal Catheter [MASC], Ablation Frontiers), and linear ablation (Tip-Versatile Ablation Catheter [TVAC], Ablation Frontiers) (see Fig. 7-4).15 One advantage of this approach is the simultaneous application of RF energy across an electrode array, intended to create contiguous lesions near an anatomical structure. Additionally, the multielectrode catheters allow selective mapping and ablation through any or all electrodes as required. This system is still undergoing investigation. Another novel ablation catheter system delivers RF energy through a flexible mesh electrode (C.R. Bard, Inc., Billerica, Mass.) that is deployed in the PV ostium. This system is undergoing investigation for PV isolation.16
Monitoring Radiofrequency Energy Delivery
The goal of optimizing RF ablation is to create an adequate-sized lesion while minimizing the chance of an impedance increase because of coagulum formation at the electrode itself, or steam formation within the tissue. As discussed previously, for ablation to be effective, power must be increased sufficiently to achieve temperatures at the tissue directly in contact with the ablating electrode that are substantially higher than 50°C to achieve tissue necrosis. At the same time, for ablation to be safe, the highest tissue temperature must be maintained at less than 100°C to prevent steam pops. Monitoring of RF energy delivery is therefore very important to help achieve successful as well as safe ablation.9
Lesion creation is influenced by many factors, some of which can be controlled, whereas others are variable and can be unpredictable. With standard RF, power delivery is titrated to electrode temperature, typically at 55° to 65°C. Higher temperatures can increase the chance of reaching 100°C at edges of the ablation electrode, thus resulting in coagulum formation. An increase in tissue temperature is accompanied by a decrease in impedance, also a reliable marker of tissue heating. Impedance reduction and temperature rise correlate with both lesion width and depth; maximum temperature rise is best correlated with lesion width, and maximum impedance reduction is best correlated with lesion depth.3,7
In addition to impedance and catheter tip temperature monitoring, reductions in amplitude and steepness of the local electrogram are important indicators for monitoring lesion growth. These indicators, however, apply only to the unipolar distal electrogram. With a bipolar recording, the signal from the ring electrode may dominate the electrogram, and the bipolar amplitude may theoretically even rise during ablation because of a greater difference between the signals from both electrodes.4
Some operators have used visualization of microbubbles on intracardiac ultrasound (ICE) as an indication of excessive tissue heating that is predictive of char and steam pops during ablation and that warrants reduction or termination of RF energy.6
Impedance Monitoring
The magnitude of the current delivered by the RF generator used in ablation is largely determined by the impedance between the ablation catheter and the dispersive electrode. This impedance is influenced by several factors, including intrinsic tissue properties, catheter contact pressure, catheter electrode size, dispersive electrode size, presence of coagulum, and body surface area. Impedance measurement does not require any specific catheter-based sensor circuitry and can be performed with any catheter designed for RF ablation. Larger dispersive electrodes and larger ablation electrodes result in lower impedance.3,7
The impedance drop during RF ablation occurs mainly because of a reversible phenomenon, such as tissue temperature rise, rather than from an irreversible change in tissues secondary to ablation of myocardial tissue. Therefore, impedance provides a useful qualitative assessment of tissue heating; however, it does not correlate well with lesion size.4 A 5- to 10-Ω reduction in impedance is usually observed in clinically successful RF applications, it correlates with a tissue temperature of 55° to 60°C, and it is rarely associated with coagulum formation. Larger decrements in impedance are noted when coagulum formation is imminent. Once a coagulum is formed, an abrupt rise in impedance to more than 250 Ω is usually observed.3,7
The drop in impedance as a monitoring tool has several limitations. When blood flow rates are low, blood can also be heated, and electrode impedance drops accordingly. Moreover, a large rise in tissue temperature at a small contact area and a smaller rise with better tissue contact can result in a similar drop in impedance. Inversely, similar tissue heating with different tissue contact can result in a different change in impedance. In addition, resistive heating nearby is fast, whereas conductive heating to deeper layers is relatively slow. The former, at close distance, has a much greater effect on impedance than the latter, which occurs at greater distance. Like electrode temperature rise, the drop in impedance during RF application is not a reliable parameter for estimating tissue heating and lesion growth.3,4,7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree