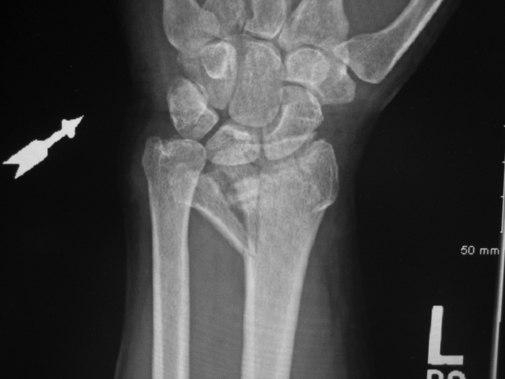
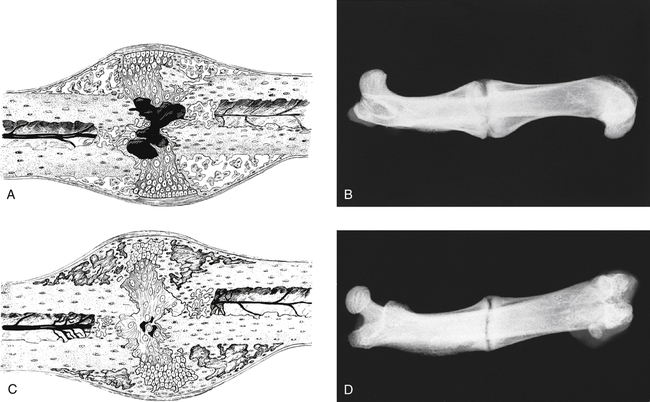
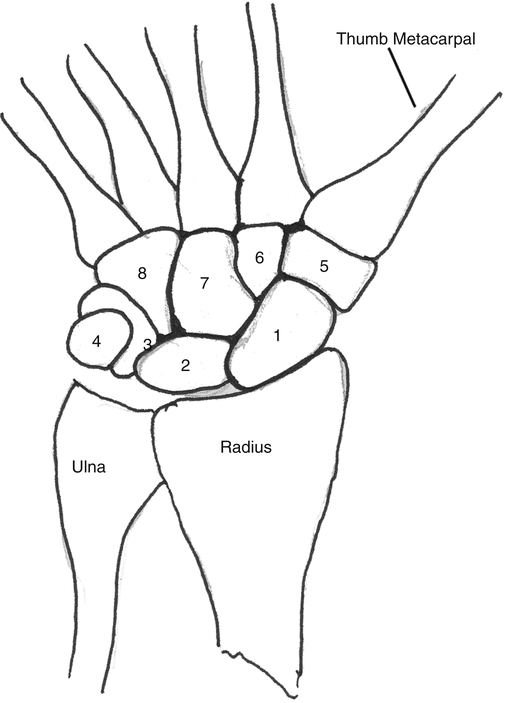
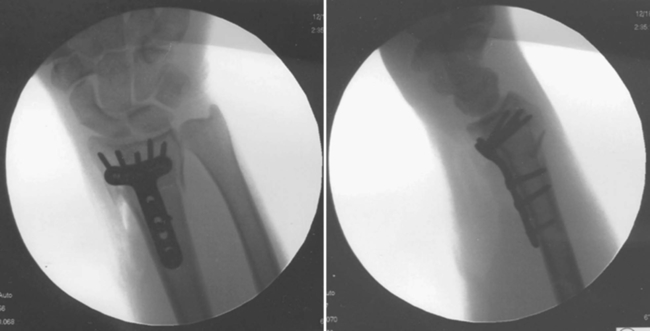
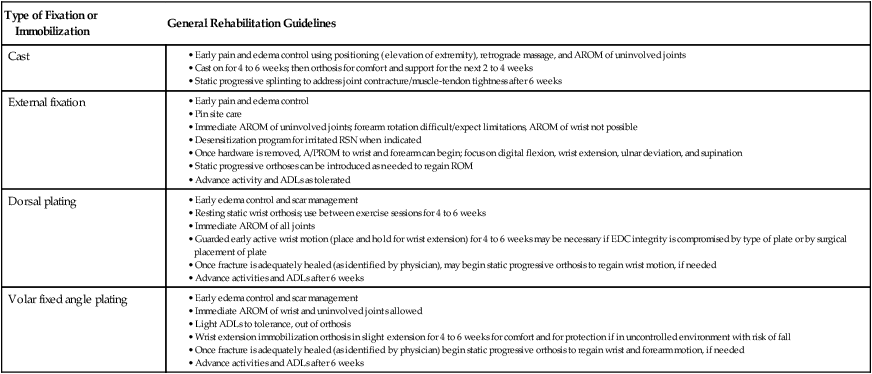
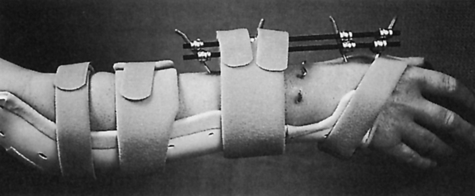
Anne M.B. Moscony and Tracy Shank “The goal of fracture healing is to regenerate mineralized tissue in the fracture area and restore mechanical strength to the bone. Ultimately, all fractures must heal with new bone, not scar tissue.”1 Following a non-displaced fracture (that is, a fracture where the periosteum remains intact), the body initiates a three-phased organized and predictable process of bone healing. The initial inflammatory phase lasts 1 to 7 days. At this time, immediate cellular and vascular responses to the injury promote the formation of a hematoma, which in turn provides some early fracture stabilization. This stage is followed by a repair phase where the damaged cells (including the hematoma) are removed and replaced with callus bone (Fig. 25-1, A and B). This soft callus is characterized by fibrous or cartilaginous tissue within the fracture gap and a significant increase in vascularity. At this point, the fractured ends are no longer freely moveable. The soft callus is gradually converted to hard callus, or woven bone tissue by a process of mineralization called enchondral ossification (see Fig. 25-1, C and D). The repair phase can last up to 4 months, although it is usually complete by 6 weeks after the injury. Finally, there is a remodeling phase where the repaired tissue is replaced and reorganized over months to years to provide the bone with its pre-injury strength and structure. This ordered process of bone tissue repair and reorganization is called secondary healing (also called callus healing, indirect healing, or enchondral ossification). Secondary fracture healing spans an average of 7 or more weeks depending on the location and type of fracture.3 The initiation of active range of motion (AROM) with these fractures depends on a number of factors, including associated soft tissue injuries and the client’s overall health and lifestyle. Often, controlled AROM can begin sometime between the third to sixth week after immobilization (that is, before there is radiographic evidence of bone healing).1,3 These fractures will continue to need intermittent protection, which is usually accomplished by an interim removable orthosis.1 By 8 to 10 weeks after the injury, most clients can begin progressive resistive exercises (or more forceful work) and leisure or homemaking tasks using the involved extremity. Resistive exercises require the healing bone to be structurally capable of tolerating high muscle forces across it.1,3 Gripping forces in particular are amplified as they cross the distal radius. Lifting heavy objects or using moderate to maximal grip strength may be enough force to disrupt bony alignment or cause plate failure. Therefore, during the first 6 weeks after surgery, clients should be restricted from gripping more than 31 psi (pounds per square inch) if using an external fixator, or 36 psi if they have a volar or dorsal plate.4,5 The therapist can teach what this amount of gripping feels like by demonstrating on the uninjured hand with the dynamometer. The introduction of resistance into the therapy plan must always be approved by the physician. A wise orthopedic surgeon observed that “…a fracture is a soft tissue injury that happens to involve the bone.”4 Fractures to the forearm are diagnosed in the emergency room by x-ray with little difficulty; however, associated soft tissue trauma may not be as easily recognized. Given the forces required to break a bone, concomitant soft tissue injuries can be expected. Sometimes these injuries heal adequately during the fracture immobilization period, and sometimes they need further rest, support, and protection requiring up to 12 weeks or more to fully heal. Soft tissue injuries commonly associated with wrist fractures may include injury of the triangular fibrocartilage complex (TFCC), peripheral nerve injury/injuries, ligament sprain or tear, and/or an aggravation of pre-existing osteoarthritis.7 Early detection of these conditions allows the therapist to explain associated symptoms to their clients, aids the therapist in determining appropriate modifications in the plan of care, and leads to more successful treatment outcomes. It is important to communicate these findings to the client’s referring physician through telephone calls and/or written reports. If surgery is required to reduce the fracture, the surgery itself produces a soft tissue disruption or “injury.” Like bone healing, soft tissue healing follows a predictable three stage process. The inflammatory phase lasts between 1 and 5 days and is characterized by a cascade of healing cells (cytokines, histamines, prostaglandins, and fibrinogen) entering the injured area. Resultant edema is usually resolved within 10 days. The second phase of soft tissue healing lasts from 2 to 6 weeks and is called the fibroblastic phase. During this phase, cells called fibroblasts polymerize, forming a diffuse collagen network of interstitial scar. Gains in range of motion (ROM) are most easily attained at this time. During the last phase, termed maturation, the scar tissue becomes more organized and acts to provide stability to the traumatized area. The maturation phase continues from 6 weeks up to 2 years. As interstitial scar becomes more organized, ROM limitations due to adhesions will become increasingly resistive to change.4 The wrist is the common term used to describe the multiple articulations that exist between the distal radius, ulna, and eight carpal bones (Fig. 25-2). The distal radius articulates with the scaphoid and lunate; and this is called the radiocarpal joint. It is a biaxial ellipsoid joint, meaning it has two axes of motion: (wrist) flexion/extension and radial/ulnar deviation. The normal radiocarpal joint has a slight palmar tilt or angulation (of about 10° to 15°) when viewed laterally by x-ray. Maintaining this palmar tilt following fracture of the radius has been linked to functional outcomes, including return of pain free normal ROM.8 The ulna does not directly articulate with the proximal carpal row. Instead, the TFCC, a hammock-like structure composed of cartilage and ligaments, suspends the ulnar carpus and acts as both a force distributor between the ulna head and triquetrum, and a primary stabilizer for the distal radioulnar joint (DRUJ) (Fig. 25-3). The central portion is an articular disc that provides a smooth gliding surface for the ulnar carpus. It has no blood supply; therefore, it will not heal if torn. The peripheral portions are ligamentous and capable of bearing tensile loads generated during gripping or weight bearing on the wrist. These portions have fair-to-excellent blood supply and accordingly have a fair-to-good capacity to heal following injury. When bearing weight on the wrist, or gripping, about 80% of the load traveling through the carpal bones is transferred to the radius and 20% is transferred to the ulna by way of the TFCC.9 Therefore, injury to the TFCC may have significant functional repercussions for our clients, including loss of grip strength and pain with loading of the wrist and/or distal forearm. The distal radius articulates with the distal ulna to form the DRUJ (Fig. 25-4). This is an important joint because it is a major site of persistent symptoms and residual disability after fracture of the distal radius.10 The DRUJ is a uniaxial pivot joint that allows rotatory motion called supination and pronation of the forearm. The action of forearm rotation involves the radius pivoting around a relatively stable ulna. However, the ulna is not completely stationary. There is a “proximal-distal” translation of the ulna with regard to the radius during pronation. In fact, the ulna slides distally up to 2 mm in relation to the radius during pronation.9 This subtle movement is relevant in that a fracture (of the radius or ulna) can result in changes in the relationship between the length of the radius and ulna and/or in how much the ulna translates or moves during pronation. It is more common to see distal radius/ulna fractures as opposed to shaft fractures because of the difference in the density of the bone. The shaft of a long bone (such as, the radius) has more cortical bone, which is denser and thus more difficult to break. It also has less blood flow and on average takes longer to heal. The enlarged distal end of the radius has more cancellous bone, which is less dense (that is, more susceptible to fracture). On the other hand, cancellous bone has a greater blood supply, which contributes to faster healing as compared to fractures of the shaft of a bone.14 A comminuted extra-articular fracture is usually a high-energy injury, resulting in the bone breaking into multiple segments. Once reduced, these fractures are secured with pins, wires, plates, and/or screws. This type of injury is less stable than those described earlier and the length and angulation of the bone’s articular surface may be altered with adverse functional ramifications. Similarly, a comminuted intra-articular fracture is a high-energy injury characterized by multiple bone fragments, some of which shift or protrude into the joint space. These are highly unstable fractures that require surgical reduction and stabilization techniques (Fig. 25-5).13 Distal forearm or wrist fractures usually (47%) result from impact to the outstretched hand and wrist during falls (Fig. 25-6). This mode of injury is sometimes referred to as a fall on out-stretched hand (FOOSH). Distal forearm fractures account for approximately 44% of upper extremity fractures11 and 15% of all fractures in adults in the United States.10 The likelihood of falls resulting in a distal radius fracture is greatest in two groups. The first group consists of those between the ages of 5 and 14 years who sustain a high-energy, sports-related fall. Increased participation in sports (such as, soccer, rugby, and snowboarding) seems to be correlated with this relatively high incidence of wrist fractures. The second group consists of seniors who sustain a low-energy fall and have resultant fracture(s) due to osteoporotic bone. The incidence of distal forearm/wrist fractures is expected to increase by 50% over the next 20 years due to the growth of the elderly population.12 There are several types of distal forearm fractures. A Colles’ fracture is the most common type; and is also one of the most common fractures seen in the human body.10,11 It is defined as a complete fracture of the distal radius with dorsal displacement of the distal fragment and radius shortening (Fig. 25-7 A). It is usually extra-articular, minimally displaced, and stable—meaning the fracture will stay reduced when placed in a cast or a fracture brace. The majority of these fractures occur in postmenopausal women with osteoporotic bone.12 See Table 25-1 for a list of common forearm fractures and their clinical features. TABLE 25-1 Common Forearm/Wrist Fractures Smith’s fracture is a complete fracture of the distal radius with volar displacement of the distal fragment (see Fig. 25-7 B). This is the second most common distal radius fracture. A Smith fracture is frequently unstable and requires some type of internal fixation to hold the displaced distal fragment in correct alignment for healing. Extra-articular, stable fractures are usually treated with closed reduction and casting for a period of 2 to 8 weeks.13,15 The position of immobilization is one of moderate wrist flexion and ulnar deviation, because this position uses the surrounding intact soft tissue to help maintain the fracture reduction. Complex distal radius fractures, such as a volarly displaced fracture-dislocation of the distal radius (a Barton’s fracture), or those fractures who have a concomitant open wound with soft tissue damage will usually require an open reduction and internal fixation (ORIF) of the fracture with a plate (Fig. 25-8). In this case, stability should be adequate to allow early active motion of the wrist, starting within the first week or so postoperatively. Control of edema, wound care and scar management, pain management, and restoration of digit motion are priorities along with return of wrist and forearm motion during the first 2 months of therapy. Close communication between the therapist and the physician is necessary for at least 8 to 10 weeks postoperatively.4 The internal rigid stability offered by a plate and/or screws serves as a substitute external callus, providing the motionless environment required for bone healing. The many advantages to rigid fixation of a fracture include avoidance of callus formation with potential local tissue adherence and early initiation of motion (within 1 week of surgery). A significant disadvantage is that plates or screws, particularly dorsally-placed ones, can cause long-term client discomfort and problems with tendon gliding, inability to extend the IP joint of thumb due to trapping of the EPL beneath the plate, and rubbing of the tendon against the plate may result in tendon rupture. In these cases, a secondary surgery may be necessary to remove the hardware. Other disadvantages include greater scarring and potential for infection. Finally, tensile strength development in the new bone following primary healing is not accelerated by using rigid fixation;1 therefore, one cannot introduce strengthening exercises any sooner than one would with secondary fracture healing. See Table 25-2 for a summary of guidelines with each type of medical/surgical management technique.16 TABLE 25-2 Types of Fixation Following Distal Radius Fracture and Associated Rehabilitation Guidelines Modified from Smith D, Brou K, Henry M: Early active rehabilitation for operatively stabilized distal radius fractures, J Hand Ther 17(1):43-49, 2004. The original plates came out in the early 1970s and were T-shaped and held in place by screws. Concerns developed with regard to dorsal plating, including issues with tendon adherence, tendon rupture, and tenosynovitis. This has led to the development of lower profile designs. Bone fragments, while stabilized via screws and/or a plate, require time for the fragments to unite firmly by new bone. New bone remodels over time with increased fracture site stiffness that will gradually mature such that the bone is able to withstand increased loads during gripping and lifting. Mature bone requires exposure to regular mechanical stress to maintain optimal health and strength. However, plates do not get stronger when exposed to mechanical stress; rather they will weaken and eventually bend or break once the metal fatigues. Therefore bone beneath a plate must have adequate strength to absorb external loading forces before much resistance is introduced to the injured area. Slutsky and Herman4 discuss fracture site forces that can disrupt a plate, noting that grip forces during therapy should remain less than 159 N (less than 36 lbs) during the initial four weeks post fracture plating of the distal radius. Given how difficult it would be to explain to our clients just how to limit their grip forces—both at home and in the clinic—it is best to introduce strengthening exercises only after the physician has seen radiographic evidence of bone healing under the plate. “Despite a lack of compelling clinical data proving its superiority, open reduction and volar locked plate fixation of distal radius fractures has increased in popularity in recent years. External fixation remains, however, a viable treatment option for patients with distal radius fractures. In particular, external fixation can be useful as an adjunct to pin fixation in fractures with significant comminution in which direct fixation or buttressing of the fracture fragments cannot be obtained, or as temporary or definitive fixation in fractures associated with severe soft tissue contamination or vascular injury.”2 If traction across the fracture site cannot be achieved with a plate and screws, an external fixator may be used. Various types of external fixators are commercially available. These devices consist of pins, wires, or screws that attach the appropriately aligned and stabilized injured bone to an external low profile scaffold. Tension can be adjusted relatively easily by the physician. Reduction by means of an external fixator allows the therapist access to the hand and wrist for hygiene and edema control. Without a bulky cast, the client may have an easier time performing AROM of uninvolved adjacent joints. These devices are removed after a period of 4 to 6 weeks, once the risk of loss of reduction is resolved or minimized. At this point, wrist ROM can begin. There is a high complication rate associated with these devices. Complications include median neuropathies, irritation of the dorsal sensory branch of the radial nerve, damage to finger and/or wrist tendons, finger stiffness, musculotendinous tightness, and most commonly, pin tract infections.15 If the client has an external fixator, a removable forearm based ulnar wrist orthosis to support the ulnar wrist and the mobile transverse arch of the hand (Fig. 25-9), will improve client comfort. Over 50% of the time, fractures of the ulnar styloid occur in conjunction with distal radius fractures; however, these ulnar styloid fractures do not typically require formal medical intervention. They do not tend to impede function or cause lasting discomfort for the client, even though many of these go on to a non-union.9 While a fracture of the distal tip of the ulnar styloid typically poses no long term problems, it is helpful to be aware of its presence or absence in explaining early-on versus later-developing ulnar sided wrist pain. If the client is aware of the presence of an ulnar styloid fracture, it may be helpful to reassure him that these fractures are usually treated with benign neglect and that early ulnar-sided wrist pain may be indicative of nothing more than the body’s attempt to assimilate to this fracture. A wrist immobilization orthosis that stabilizes the ulnar side of the wrist may help manage the pain associated with these fractures. A fracture to the proximal or middle one-third of the ulnar styloid may involve the TFCC, and possibly the insertion of the ECU tendon. If the fracture is non-displaced, a long arm cast or brace is worn for 3 to 4 weeks to prevent wrist and forearm motion until the fracture is healed. If the fracture is displaced, ORIF is necessary, followed by immobilization in a long arm cast for about 3 to 4 weeks.9,16 Distal ulnar shaft fractures can also disrupt the DRUJ; these may require either open or closed reduction and some type of fixation (for 4 to 6 weeks) to achieve stability.9
Wrist Fractures
General Timelines and Healing
Associated Soft Tissue Injuries Following Bone Fracture
Distal Forearm Fractures
Anatomy
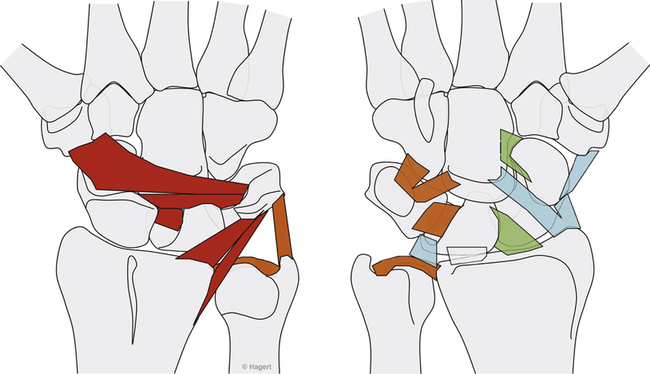
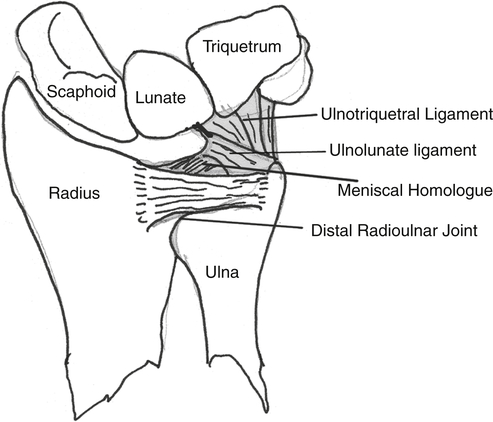
The head of the ulna covers 80% of the surface and articulates with the sigmoid notch of the distal radius (radially) and with the inferior surface of the triangular fibrocartilage complex (TFCC). L, Lunate; S, scaphoid; T, triquetrum. (Copyright the Mayo Foundation. From Cooney WP, Linscheid RL, Dobyns JH, editors: The wrist: diagnosis and operative treatment, St Louis, 1998, Mosby, p. 775.)
Diagnosis and Pathology
Distal Forearm Fractures
Type of Fracture
Clinical Feature
Colles’ fracture (see Fig. 25-6, A)
A fracture of the distal radius with dorsal displacement (from hyperextension of the wrist). Managed surgically with closed reduction and casting or ORIF with volar plate and sometimes simultaneous carpal tunnel release as indicated. Will typically need temporary orthosis for support and protection and some therapy to regain full ROM.
Smith’s fracture (see Fig. 25-6, B)
A fracture of the distal radius with volar displacement (from hyperflexion of wrist). Surgical and therapeutic management is same as Colles’ fracture.
Barton’s fracture
Fracture-dislocation of rim of radius along the carpus caused by shearing forces from the proximal carpus translating across the radius. Surgical management with plate or screw. Therapy indicated to regain ROM and for protective orthosis.
Chauffeur’s fracture
Fracture of the radial styloid. Typically surgically managed with pinning. Minimal therapy needs. Check for irritation of DRSN.
Salter-Harris fracture
Fracture of the growth (epiphyseal) plate of children and teens. At least nine variations/types have been described. These injuries are potentially serious and necessitate a visit to a hand surgeon versus general practitioner and may require some follow-up by a hand therapist.
Galeazzi’s fracture
Unstable fracture of radial shaft and DRUJ disruption. Usually the result of a FOOSH and seen most often in males. Need to check for AIN palsy and trauma to the radial nerve. Higher risk of malunion than other forearm fractures. Usually treated with closed reduction in children and always with ORIF in adults due to the otherwise chronic dislocations of the ulna. Requires skilled therapy post-surgery.
Monteggia’s fracture
Unstable fracture of ulnar shaft and radial head dislocation. Usually the result of a FOOSH or blunt trauma (nightstick injury). In children these usually heal readily with closed reduction and casting. In adults, ORIF is typically preferred and skilled therapy is required due to high risk of complications.
Greenstick fracture
Incomplete fracture common in children. Concave side of bone may be intact or buckled. Convex side has fracture. Typically casted for 3 to 5 weeks and does not require therapy.
Torus (buckle) fracture
Incomplete fracture common in children. Concave side of bone compresses (buckles). Convex side is intact. Pain and/or swelling occurs at the fracture site. Movement may be painful. Typically casted for 3 to 5 weeks. Therapy is frequently not needed.
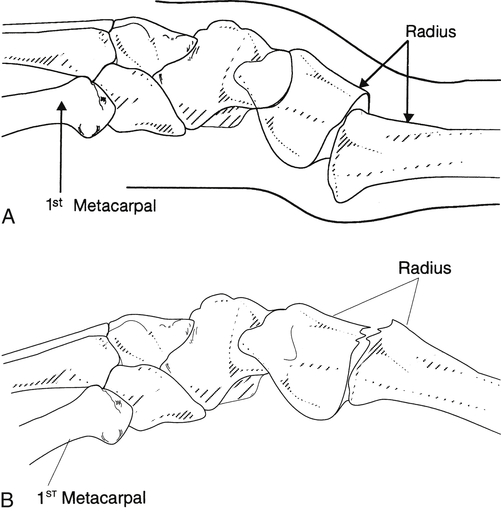
A, Colles’ fracture with dorsal displacement of distal fragment. B, Smith’s fracture with volar displacement of distal fragment. (From Stanly BG, Tribuzi SM, editors: Concepts in hand rehabilitation, Philadelphia, 1992, FA Davis.)
Non-Operative Treatment for Distal Radius Fractures
Operative Treatment for Distal Forearm Fractures
Diagnosis-Specific Information That Affects Clinical Reasoning
Wrist Fractures