CHAPTER 12 Venous ulcers
1. Discuss venous ulcers in terms of etiologic factors, risk factors, assessment, diagnostic criteria, pathophysiology, typical presentation, and principles of management.
2. Describe Laplace’s law in predicting sub-bandage pressure and the level of compression applied to the lower leg.
3. Explain the mechanism of action underlying effective compression therapy for the individual with chronic venous insufficiency.
4. Discuss considerations for use of inelastic compression, short-stretch bandages, long-stretch bandages, compression stockings, and intermittent pneumatic compression.
5. Identify adjunctive therapies that may be of benefit to the patient with a venous ulcer.
6. List three key points to teach a patient being treated with a multilayer compression wrap.
Chronic venous disorders include a wide range of morphologic and functional abnormalities of the venous system, that includes mild conditions (e.g., uncomplicated telangiectasias and varicose veins) to complex conditions (e.g., deep vein thrombosis, and venous ulcers). The majority of chronic venous disorders exist in the healthy patient population. The term chronic venous disease is used when referring to the subset of chronic venous disorders that are more complicated; those that are associated with signs and symptoms significant enough to require medical care, such as deep vein thrombosis, chronic venous insufficiency, and venous ulcers. Lower extremity venous disease (LEVD) is synonymous with chronic venous disease. Chronic venous insufficiency (CVI) refers to leg-related manifestations of venous hypertension and functional abnormalities of the venous system (edema, skin changes, ulceration) (Meissner, 2009). Venous ulcers are the most common lower extremity ulcers, accounting for 70% to 90% of all leg ulcers (WOCN Society, 2005). These lesions develop as a result of skin and tissue changes caused by CVI and the associated ambulatory venous hypertension.
Management of patients with venous ulcers must include measures to optimize wound healing through reduction of edema, prevention of complications, and appropriate topical therapy to promote healing (de Araujo et al, 2003; Moffat et al, 2007; Robson et al, 2006). Once the ulcer is healed, the emphasis shifts to long-term disease management and prevention of recurrence.
Epidemiology
The exact prevalence of venous ulcers is not known, although prevalence estimates in developed countries range from less than 1% to greater than 3% of the population (Bolton, 2008; Kerstein, 2003). Venous disease and venous ulcers occur in individuals as young as 20 years. “Peak” incidence occurs between the ages of 60 and 80 years (de Araujo et al, 2003). Although no racial predilection is apparent, most studies report female gender is a risk factor (de Araujo et al, 2003; Kalra and Gloviczki, 2003). In addition, an increased incidence of obesity is seen in 25% of patients with venous ulcers (Benigni et al, 2006).
LEVD affects approximately six to seven million individuals in the United States, and approximately one million of these persons will develop ulcerations (WOCN Society, 2005). The impact of venous disease is tremendous as it relates to the individual and costs to the health care system and society. Individuals with venous disease report pain, itching, anxiety, social isolation, and reduced ability to perform usual activities as their areas of greatest concern (de Araujo et al, 2003). In contrast, nurses caring for these patients rated pain control as a less important aspect of care than wound healing and limb preservation, which indicates the need for increased awareness and focus on quality-of-life issues on the part of health care providers (Ryan et al, 2003).
Approximately $2.5 to $3.5 billion is spent annually on management of venous ulcers (Bolton, 2008). The average lifetime cost of care for an individual with LEVD can exceed $40,000 (Weingarten, 2001). Cost per occurrence in the home care or wound clinic setting is estimated at $1,621 to $3,279 without calculating lost wage time (Bolton et al 2006; Korn et al, 2002; McGucklin et al, 2002).
The negative impact of venous ulcers is compounded by recurrence rates of 26% to 28% in the first year and is as high as 76% within 3 to 5 years, which reflects the chronicity of the underlying condition (Bolton et al, 2006; Castonguay, 2008) Frequent recurrence is attributed to a failure to adequately address the primary problems of venous insufficiency and venous hypertension (WOCN Society, 2005).
Venous structure and function
The veins of the lower extremity venous system include deep veins, superficial veins, and perforator veins. The deep veins include the posterior and anterior tibial and the peroneal veins; these veins are located in the deep tissue adjacent to the calf muscle. The superficial venous system is also known as the saphenous system because the two major vessels are the greater saphenous vein and lesser saphenous vein. These two vessels are located just below the superficial fascia and have multiple tributaries located in the superficial tissues (Figure 12-1) (Kalra and Gloviczki, 2003). The perforator veins “connect” the two systems, transporting blood from the superficial system into the deep system, from which point the blood is propelled back to the heart. The number of perforator veins per leg can vary greatly; the typical patient can have 200 perforator veins below the knee and 20 above the knee (Hussein, 2008).
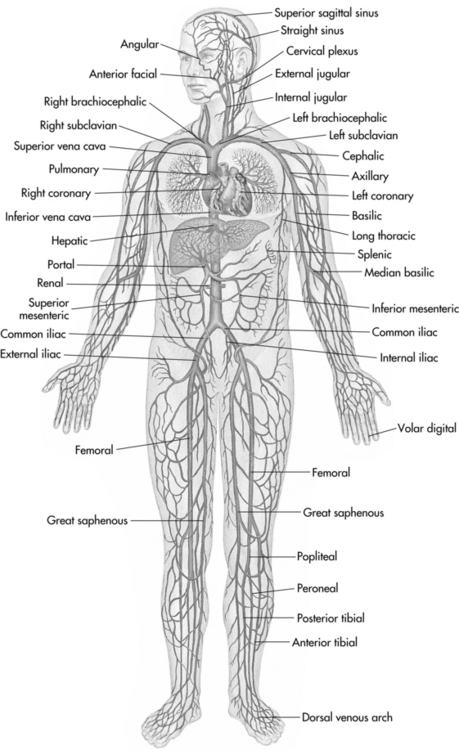
FIGURE 12-1 Systemic circulation: veins.
(From Seidel HM et al: Mosby’s guide to physical examination, ed 6, St. Louis, 2006, Mosby.)
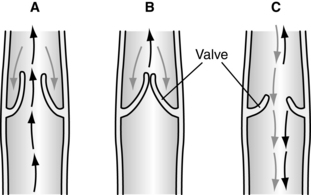
Veins fill normally via slow capillary inflow, which takes more than approximately 20 seconds. All veins are equipped with one-way valves that support a unidirectional flow of blood toward the heart. Because these valves prevent reflux of blood from the high-pressure deep venous system to the low-pressure superficial venous system, they play an essential role in normal venous function. Further protection is provided by the fact that the perforator veins follow an oblique course through the fascia and muscle layers, which provides additional support for the connecting veins and their valves. The closed valves in the perforator veins prevent transmission of the high resting pressures back into the superficial system, so long as the valves remain competent (Kalra and Gloviczki, 2003). Approximately 50% to 60% of patients with venous ulcers have incompetent superficial and perforator vein valves (Agren and Gottrup, 2007).
Returning blood from the feet and legs to the heart is a major physiologic challenge because the blood must flow “uphill” against the forces of gravity. When an individual is standing upright, the gravitational force creates a column of hydrostatic pressure of approximately 90 mm Hg at the ankle. The primary mechanisms by which venous blood is returned to the heart are the smooth muscle tone within the venous walls, the contraction of the calf muscles (gastrocnemius and soleus), and the negative intrathoracic pressure created during inspiration. Of these three mechanisms, contraction of the calf muscle pump is by far the most essential (Meissner, 2009).
The calf muscle pump and one-way valves normally work together to propel venous blood back toward the heart. Calf muscle contraction forces the blood out of the deep veins and into the central circulation. While blood is being pumped from the deep veins, the one-way valves in the perforator system are closed to prevent backflow of the venous blood into the superficial veins. As the calf muscle relaxes, the valves in the perforator veins open to permit the blood in the superficial system to flow into the deep veins. At the onset of calf muscle contraction, the pressures within the deep venous system peak at 120 to 300 mm Hg. These pressures then fall rapidly as the veins empty and the calf muscle relaxes (Figure 12-2). Thus high resting (filling) pressures, but low walking (emptying) pressures, characterize normal venous function (Figure 12-3).
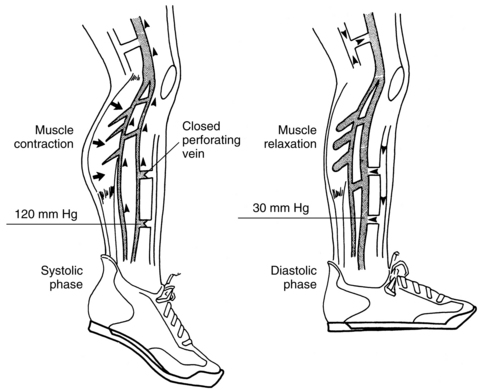
(From O’Donnell TF Jr, Shepard AD: Chronic venous insufficiency. In Jarrett F, Hirsch SA, editors: Vascular surgery of the lower extremities, St. Louis, 1996, Mosby.)
Chronic venous insufficiency
Valve failure changes the normal unidirectional flow of blood into a “bidirectional” flow. As a result, blood refluxes back into the superficial system, causing distention and congestion of the superficial veins and capillaries, which manifest clinically as edema. The deep veins are incompletely emptied, causing increased pressures within the deep system, which create resistance to blood draining from the superficial veins. Resistance to flow creates congestion and distention of the superficial and perforator veins, which cause loss of valve coaptation. The incompetent valves then permit backward transmission of the high pressures in the deep system (Meissner, 2009). The failure to adequately lower venous pressure with the pumping of the calf muscle or by the incompetence of the valves creates ambulatory venous hypertension. Venous ulceration is a direct result of ambulatory venous hypertension from CVI. A clear understanding of the anatomy and physiology of the lower extremity venous system provides the framework for determining the pathology of LEVD, ambulatory venous hypertension, and venous ulceration.
The majority of patients have multisystem valvular incompetence (i.e., incompetent valves in at least two of the three venous systems) (Meissner, 2009). Perforator valve incompetence is particularly common and clinically significant. At least two thirds of patients with venous hypertension and venous ulcers have incompetent perforator valves, which can result in supramalleolar pressures well above 100 mm Hg and a “reflux rate” greater than 60 ml/min. When multiple valves become incompetent, the effect is magnified and clinically evident disease becomes much more likely (Kalra and Gloviczki, 2003; Meissner, 2009).
Of the three leg muscle pumps responsible for venous return in lower extremities (foot, calf, and thigh), the calf muscle pump is of greatest importance and generates the highest pressure. Among the venous pumps, the ejection fraction of the calf muscle pump is 65% compared to only 15% from the thigh muscle. In the limb with active ulceration, the ejection fraction can decrease to 35% (Meissner, 2009).
Ultimately, the end result of prolonged ambulatory venous hypertension is damage to the skin and soft tissues that renders these structures vulnerable to minor trauma and susceptible to spontaneous ulceration. Venous ulcers are caused primarily by chronic valvular disease of the deep venous system and perforators (Hussein, 2008). In the past, the cutaneous inflammation observed with venous insufficiency was believed to the result of blood pooling (thus the term stasis) with low oxygen tension in the superficial veins, which precipitated hypoxic damage to the overlying skin. Today, no evidence supports the theories of stasis or hypoxia, prompting discontinuation of the terms stasis dermatitis and stasis ulcers (Flugman and Clark, 2009).
Classification
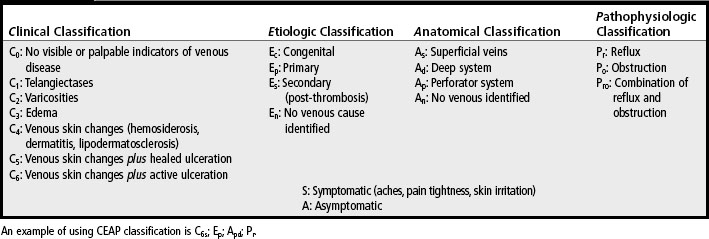
CVI is classified according to: Clinical indicators, Etiologic factors, Anatomic location of the dysfunctional venous structures, and specific Pathophysiologic processes (CEAP). This system is presented in Table 12-1 (Agren and Gottrup, 2007; WOCN Society, 2005).
Risk factors
Risk factors for CVI include a history of major leg trauma, hip or knee surgery, and vein stripping. However, factors that lead to valvular or calf muscle dysfunction are the most common risk factors; they are listed in Table 12-2 along with other key elements of the medical history that must be obtained for the patient with a venous ulcer.
TABLE 12-2 Key Elements of History for Patients with Venous Ulcers
| Element | Description |
|---|---|
| Risk factors for valvular dysfunction | Obesity, pregnancy, thrombophlebitis, leg trauma (e.g., fracture), thrombophilic conditions |
| Risk factors for muscle dysfunction | Sedentary lifestyle, prolonged standing, advanced age, altered or “shuffling” gait, musculoskeletal conditions and surgeries that compromise calf muscle function (e.g., paralysis, arthritis) |
| Factors that impede healing | Diabetes, tobacco, malnutrition, unplanned weight loss, medications |
| Factors that impede treatment | Limited activity and mobility, cardiac disease, heart failure (clinically significant heart failure is contraindication to compression) |
| Ulcer history | Previous ulcers, onset, duration, precipitating event (duration >6 months is negative predictor for wound healing) |
| History of prior treatment | Surgical, pharmacologic, compression (venous ulcers that consistently fail to respond to treatment should be evaluated for misdiagnosis, malignant or mixed disease) |
| Patient concerns and anticipated barriers | Pain, itching, anxiety, anticipated barriers, transportation, ability to apply compression, job/financial limitations, impact on activities of daily living, treatment goals and priorities |
Valvular dysfunction.
Numerous risk factors for valvular dysfunction have been identified and include the following (Burrows et al, 2007; WOCN Society, 2005):
• Obesity, which creates resistance to venous return due to pressure on pelvic veins
• Pregnancy, especially multiple pregnancies or pregnancies that are close together, because of increased pressure against pelvic veins and compromised venous return
• Thrombophlebitis (e.g., deep vein thrombosis, pulmonary embolism), which triggers an inflammatory response that can cause direct damage to the valve leaflets, or chronic partial deep vein obstruction due to incomplete recanalization of the vein, which in turn causes venous distention and valvular compromise
• Leg trauma (e.g., fracture), which suggests undiagnosed damage to the vessel walls and valves
• Thrombophilic conditions (e.g., protein S deficiency, protein C deficiency, factor V [Leiden mutation]), which increase the coagulability of venous blood, thus increasing the risk of deep vein thrombosis and microvascular thrombosis; thrombophilic conditions have been identified in as many as 50% of patients with venous ulcers
Calf muscle dysfunction.
The dynamics of the calf muscle pump can be adversely affected by changes that accompany major injuries, neurologic disease, and bone or joint pain. The calf muscle becomes weak with disuse; gait changes can exacerbate venous hypertension and calf muscle atrophy (Burrows et al, 2007). Risk factors for compromised calf muscle function include the following:
• Occupations that require prolonged standing
• Musculoskeletal conditions that compromise calf muscle function (e.g., paralysis, arthritis)
• Advanced age, which is associated with decreased elasticity of the calf muscle tendon
• Altered or “shuffling” gait that fails to induce calf muscle contraction; reduced mobility and gait do not relate to calf muscle dysfunction
• Arthroscopic surgery, which could cause fixation of the hip, knee, or ankle, leading to loss of calf muscle pump
• Injection drug use due to progressive deterioration of the venous function of the legs (Pieper et al, 2008)
Pathology of venous ulceration
Fibrin cuff theory.
Browse and Burnand (1982) initially postulated that capillary bed distention permitted leakage of large molecules such as fibrinogen into the dermal tissue, and that the fibrinogen then polymerized to form a thick perivascular cuff composed of fibrin, fibronectin, laminin, tenascin, and collagen. These cuffs do not pose any barrier to the diffusion of oxygen and nutrients into the tissues; therefore skin hypoxia cannot be the ultimate factor in the pathogenesis of venous ulcerations (Meissner, 2009).
White blood cell activation and trapping theory.
The trap hypothesis suggests that venous hypertension reduces the velocity of blood flow in the postcapillary bed. When blood flow becomes sluggish, leukocytes begin to adhere to each other (leukocyte aggregation) and/or to the capillary walls (leukocyte margination). This triggers the release of toxic oxygen metabolites, proteolytic enzymes, and cytokines causing tissue inflammation and dermal fibrosis (Meissner, 2009). Dermal capillary loops also become plugged with leukocytes so that fibrin and other macromolecules leak out of the permeable capillary beds into the dermis, further aggravating inflammatory and fibrotic changes in the subcutaneous tissues and rendering them very susceptible to ulceration, which can occur spontaneously or as a result of minor trauma. Leukocyte migration and activation and the interaction of leukocytes with the endothelium in the presence of venous hypertension play considerable roles in the pathophysiology of venous ulcerations (Agren and Gottrup, 2007; Kalra and Gloviczki, 2003; WOCN Society, 2005).
Assessment
Key assessment parameters for patients with a leg ulcer include medical history, ulcer history, previous treatments, clinical examination, inspection of the ulcer, and Doppler assessment of pulses (Nelzen, 2007). Assessment of Doppler pulses are discussed in Chapters 10 and 11 and illustrated in Figure 11-1.
Patient history
Risk factors for CVI are identified through the patient history. Of particular importance are risk factors that differentiate venous insufficiency from arterial disease and other pathologies that may cause ulceration in the lower extremity (see Table 12-2). Because of the contraindications to sustained compression, pretreatment evaluation must include a cardiac history and any indicators of uncompensated heart failure.
Lower extremity assessment
Both legs need to be examined by the clinician to ascertain if the manifestations are bilateral or more severe on one extremity. Findings unique to LEVD include edema, hemosiderosis, dermatitis, lipodermatosclerosis, atrophie blanche, varicose veins, ankle flaring, and scarring from previous ulcers (WOCN Society, 2005). Checklist 12-1 gives a list of assessment findings unique to the leg of a patient with CVI.
Arterial perfusion.
Concomitant arterial disease occurs in as many as 25% of patients with venous ulcers (de Araujo et al, 2003; Ryan et al, 2003). Therefore an ankle-brachial index (ABI) should be obtained to determine if some degree of arterial insufficiency is present. An ABI of 1.0 indicates a “pure” venous ulcer (no coexisting arterial insufficiency). An ABI of 0.9 or less indicates arterial insufficiency is present; these ulcers are referred to as mixed arterial/venous ulcers. This is an important initial assessment because compression (critical for treatment of venous ulcers) must be modified or, in some cases, omitted. Wounds that begin specifically as a venous ulcer can develop an arterial component, so monitoring for signs of arterial disease at regular intervals is necessary (WOCN Society, 2008).
Edema.
Edema is a classic indicator of venous insufficiency because of the combination of capillary bed distention and elevated intracapillary pressures. As described in Chapter 10, the severity of edema varies among patients and from time to time throughout the day. The classic pattern is pitting edema (see Figure 10-1) that worsens with dependency and improves with elevation. Box 10-1 describes the assessment of pitting edema. With prolonged disease and gradual fibrosis of the soft tissues, edema may become “brawny,” that is, nonpitting. Thus the characteristics of the edema are a clue to the duration of the underlying disease process.
The distribution of edema is also indicative of the underlying process. Venous edema primarily involves the lower leg between the ankle and the knee. In contrast, lymphedema and lipedema involve the entire extremity. Table 10-1 gives a comparison of the edema associated with these three conditions. Measuring the circumference of the calf and gaiter area is another method for assessing edema, especially if it is unilateral. Thus changes in these circumferential measurements provide an indication of the effectiveness of compression therapy (Nelzen, 2007). Circumferential measurements usually are taken weekly when the compression bandage is changed.
Hemosiderin staining (hemosiderosis).
Another “classic” indicator of venous insufficiency is hemosiderosis, the discoloration of the soft tissue located in the gaiter area that results when extravasated red blood cells break down and release the pigment hemosiderin. The result is a gray-brown pigmentation of the skin known also as hyperpigmentation or tissue staining (de Araujo, et al, 2003) (see Plate 34). Hemosiderin plays a role in the evolution of skin changes toward lipodermatosclerosis and ulceration (Caggiati et al, 2008).
Lipodermatosclerosis.
Lipodermatosclerosis (see Plate 38), a term used to denote fibrosis, or “hardening,” of the soft tissue in the lower leg, is indicative of long-standing venous insufficiency. The fibrotic changes typically are confined to the gaiter, or “sock,” area of the leg, which results in an inverted “champagne bottle” or “apple core” appearance of the affected lower leg. The fibrosis causes abnormal narrowing of the affected area, which contrasts sharply with the normal tissue in the proximal limb, and a “woody,” hard texture when the area is palpated. These fibrotic changes are thought to result from a combination of fibrin deposits, compromised fibrinolysis, and deposition of collagen in response to growth factors produced by activated white blood cells (de Araujo et al, 2003). A body mass index greater than 34 has been found to predispose to lipodermatosclerosis (Bruce et al, 2002).
Varicosities.
Varicose veins are swollen and twisted veins that appear blue, are close to the skin’s surface, may bulge or throb, cause the legs to swell, and precipitate a feeling of heaviness. They are most often seen in the back of the calf or the medial aspect of the leg. Varicosities precede valvular incompetence and appear to develop as a consequence of intrinsic structural and biochemical abnormalities of the vein wall (Meissner, 2009). Patients with varicosities should manage their weight and exercise and avoid crossing their legs and wearing constrictive garments (WOCN Society, 2005).
Skin changes near the ankle.
Ankle blowout has been described as uncommon painful clusters of tiny venous ulcers located near the medial malleolus originating from dilated ruptured vessels (Kunimoto, 2001), but no further discussion related to ankle blowout has been noted in recent literature or evidence-based guidelines. Malleolar flare has been described in recent guidelines as visible capillaries from distention of small veins around the medial malleolus (WOCN Society, 2005).
Atrophie blanche lesions.
Atrophie blanche lesions (see Plate 35) can be found in as many as one third of patients with LEVD. These lesions are smooth white plaques of thin, “speckled” atrophic tissue with tortuous vessels on the ankle or foot with hemosiderin-pigmented borders. Sometimes mistaken for scars of healed ulcers, this clinical finding actually represents spontaneously developing lesions. Prompt recognition is important so that a plan can be established to protect these high-risk areas from ulceration due to the thin, atrophic epidermis (de Araujo et al, 2003; Ryan et al, 2003; WOCN Society, 2005). Ulcers occurring in this area usually are small, very painful, and hard to heal. Topical steroids should be avoided because they can cause further damage to the very fragile skin.
Venous dermatitis.
Venous dermatitis is a common but distressing inflammation of the epidermis and dermis on the lower extremity of the patient with LEVD (see Plate 38). Often the earliest cutaneous sequelae of venous insufficiency, they most commonly affect middle-aged to elderly patients (Flugman and Clark, 2009). Venous dermatitis is characterized by scaling, crusting, weeping, erythema, erosions, and intense itching; symptoms may be acute or chronic. The cutaneous inflammation of venous dermatitis is often confused with cellulitis. Factors that distinguish between dermatitis and cellulitis are listed in Table 12-3 (WOCN Society, 2005).
TABLE 12-3 Distinguishing Between Dermatitis and Cellulitis
| Dermatitis | Cellulitis | |
|---|---|---|
| Symptoms | Afebrile Itching Varicose veins/deep vein thrombosis | May have fever Painful No relevant history |
| Signs | Normal temperature Erythema, inflammation May be tender Vesicles and crusting Lesions on other parts of the body (e.g., other leg, arms) May be unilateral or bilateral | Elevated temperature Erythema, inflammation Tenderness One or a few bullae No crusting No lesions elsewhere Unilateral |
| Portals of entry | N/A | Usually unknown; breaks in skin, ulcers, trauma, tinea pedis, intertrigo implicated |
| Laboratory | Normal white blood cell count Negative blood cultures Skin swabs (Staphylococcus aureus common) | Leukocytosis Blood cultures usually negative Skin swabs usually negative except for necrotic tissue |
DVT, Deep vein thrombosis; WBC, white blood cell.
From Wound, Ostomy and Continence Nurses Society (WOCN Society): Guideline for management of patients with lower extremity venous disease, WOCN clinical practice guideline series #1, Glenview Ill, 2005. Data from Quartey-Papafio CM: Lesson of the week: importance of distinguishing between cellulitis and varicose eczema of the leg, BMJ 318(7199):1672-1673, 1999.
Venous dermatitis results from the release of inflammatory mediators from activated leukocytes that are trapped within the fibrin cuffs and surrounding perivascular space (Flugman and Clark, 2009). Dermal fibrosis, a hallmark of venous dermatitis, develops as a result of fibrin cuff formation, decreased fibrinolysis, and release of transforming growth factor-β1 (a mediator of dermal fibrosis) by the leukocytes. Potent chemoattractants (intercellular adhesion molecule-1 and vascular cell adhesion molecule-1) keep leukocytes active in the perivascular environment and perpetuate cutaneous inflammation with fibrosis. Why venous dermatitis is common among some patients but is rare among others is unclear (Ryan et al, 2003; WOCN Society, 2005).
Venous dermatitis increases the risk of developing contact sensitivity due to the presence of chronic inflammation of the skin (Flugman and Clark, 2009). Exposure to usually benign topical substances (e.g., wound exudate, skin sealants, adhesives, silver sulfadiazine) easily exacerbates venous dermatitis. Frequent contact allergens include lanolin, balsam of Peru, and fragrances (Romanelli and Romanelli, 2007). Patients also can become sensitized to rubber products contained in some compression wraps and stockings. More than 30% of patients with contact dermatitis developed sensitivity to the topical antibiotics neomycin and bacitracin (Alguire and Mathes, 2007). Although less common, sensitization of the skin to topical corticosteroids can develop, triggering an allergic contact dermatitis. When the clinical manifestations of the limb affected with venous dermatitis worsen despite appropriate topical therapy, contact dermatitis should be considered.
To prevent venous dermatitis, product ingredients should be carefully scrutinized before topical therapy is selected, and products containing sensitizers should be avoided (de Araujo et al, 2003). Skin moisturizers such as bland, perfume-free topical emollients and white petrolatum, can be used to maximize epidermal integrity. An essential component of prevention and treatment of venous dermatitis is graduated compression (discussed later in this chapter), which may require considerable patient education and encouragement due to the discomfort associated with an inflamed, edematous limb. Patients need reassurance that the discomfort should decrease as the edema resolves. Exudate absorbers such as alginates and hydrofibers are commonly indicated, often in combination with a secondary foam dressing to adequately absorb and contain exudate.
To reduce inflammation and itching, mild-potency topical corticosteroids (e.g., triamcinolone 0.1% ointment) can be used short term (i.e., 2 weeks) but sparingly because of the risk for skin atrophy. High-potency topical corticosteroids are rarely used because of the risk for skin atrophy as well as systemic absorption through open denuded skin. Systemic corticosteroids are seldom warranted for treatment of venous dermatitis (Flugman and Clark, 2009). Cool compresses with Burow’s solution (aluminum acetate) followed by an application of plain petrolatum also can be used to relieve itching. Additional remedies for venous dermatitis include Condy solution (potassium permanganate), dilute vinegar compresses, and cool tar ointment. Patients with severe or nonresponsive dermatitis should be referred to dermatology for management (Bonham, 2003; Ryan et al, 2003).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree