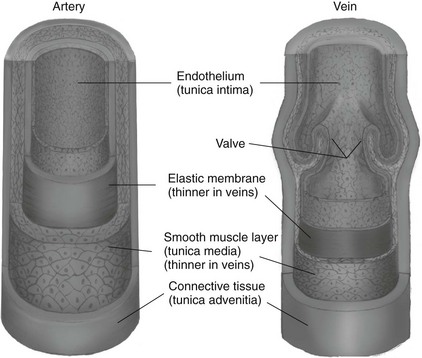
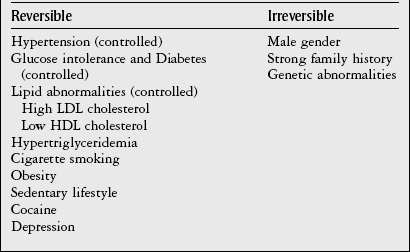
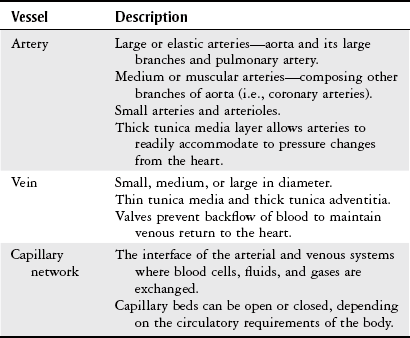
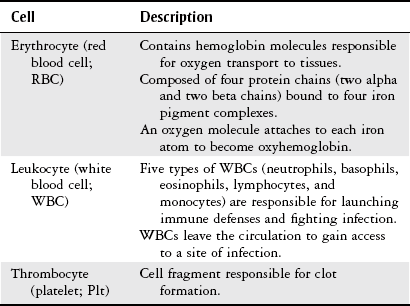
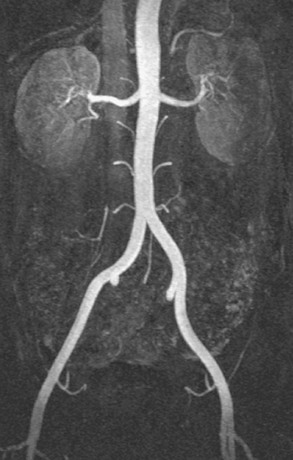
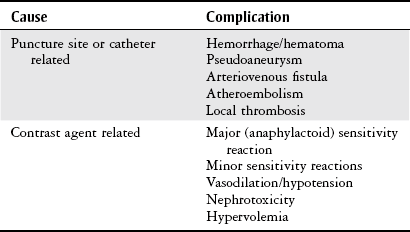
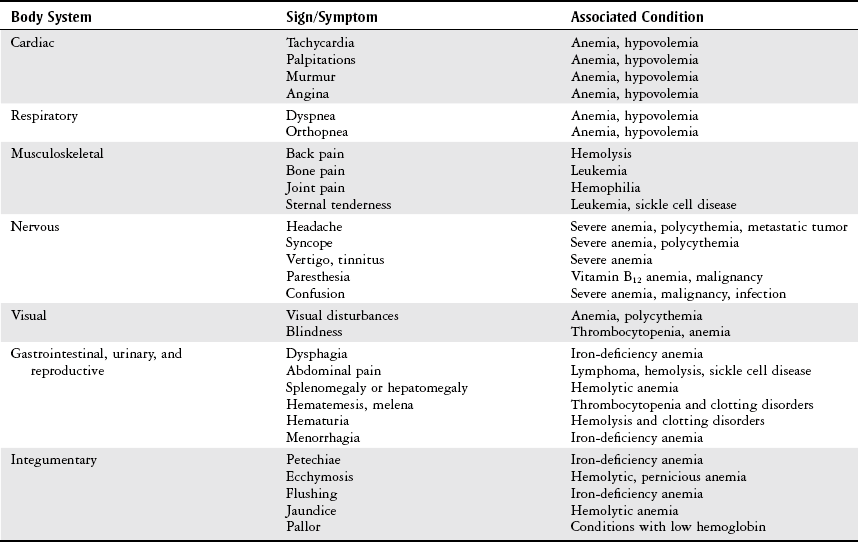
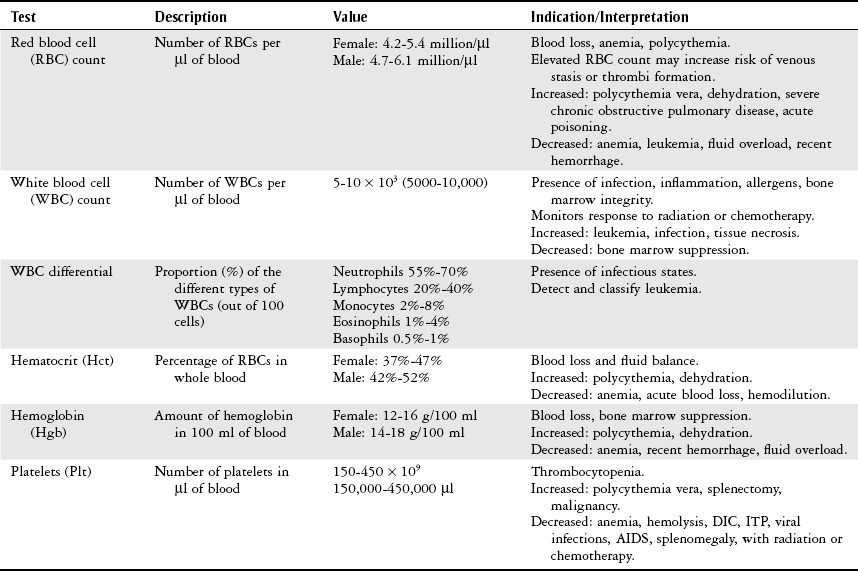
Chapter 7 The objectives of this chapter are to provide the following: 1 Review the structure and function of blood and blood vessels 2 Review the vascular and hematologic evaluation, including physical examination and diagnostic and laboratory tests 3 Describe vascular, hematologic, and lymphatic health conditions, including clinical findings, medical and surgical management, and physical therapy intervention • Arterial Disorders (Atherosclerosis, Aneurysm, Aortic Dissection, Hypertension, Raynaud’s Disease), Chronic Regional Pain Syndrome, Compartment Syndrome: 4C, 4J, 6D, 7A • Venous Disorders (Varicose Veins, Venous Thrombosis, Pulmonary Embolism, Chronic Venous Insufficiency): 4C, 6D, 7A • Combined Arterial and Venous Disorders (Arteriovenous Malformations): 4C, 6D, 7A • Hematologic Disorders (Erythrocytic Disorders [Anemia], Polycythemia), Thrombocytic Disorders: 4C, 6D Please refer to Appendix A for a complete list of the preferred practice patterns, as individual patient conditions are highly variable and other practice patterns may be applicable. The network of arteries, veins, and capillaries composes the vascular system. Living blood cells and plasma within the blood vessels are the structures that compose the hematologic system. The lymphatic system assists the vascular system by draining unabsorbed plasma from tissue spaces and returning this fluid (lymph) to the heart via the thoracic duct, which empties into the left jugular vein. The flow of lymph is regulated by intrinsic contractions of the lymph vessels, muscular contractions, respiratory movements, and gravity.1 All blood vessels are composed of three similar layers (Figure 7-1 and Table 7-1). Blood vessel diameter, length, and wall thickness vary according to location and function (Table 7-2). Note that the arteries are divided into three types, depending on their size and structural features. TABLE 7-1 Data from Marieb EN: Human anatomy and physiology, ed 3, Redwood City, CA, 1995, Benjamin-Cummings. TABLE 7-2 Characteristics of Blood Vessels Data from Marieb EN: Human anatomy and physiology, ed 3, Redwood City, CA, 1995, Benjamin-Cummings; Kumar V: Robbins and Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders. Blood is composed of living cells (Table 7-3) in a nonliving plasma solution and accounts for 8% of total body weight, or 4 to 5 liters in women and 5 to 6 liters in men. Plasma is composed almost completely of water and contains more than 100 dissolved substances. The major solutes include albumin, fibrinogen, protein globules, nitrogenous substances, nutrients, electrolytes, and respiratory gases.2 TABLE 7-3 Data from Marieb EN: Human anatomy and physiology, ed 3, Redwood City, CA, 1995, Benjamin-Cummings. The lymphatic system includes lymph vessels, lymph fluid, and lymph tissues and organs (lymph nodes, tonsils, spleen, thymus, and the thoracic duct). The lymphatic system is parallel to and works in concert with the venous system. Lymphatics are fragile and are more likely to collapse under pressure than the veins. Lymphatics are located in all portions of the body except the central nervous system and cornea. Lymph moves throughout the body through a number of mechanisms, and the excess lymph is transported to the thoracic duct and emptied into the jugular vein trunks. Lymph fluid is first absorbed at the capillary level, then channeled through the small vessels and finally picked up by the larger valved vessels.3 The function of the blood vessels is to carry blood throughout the body to and from the heart (Table 7-4). Normal alterations in the vessel diameter will occur, depending on circulating blood volume and the metabolic needs of the tissues. TABLE 7-4 Data from Nettina S: The Lippincott manual of nursing practice, ed 8, Philadelphia, 2005, Lippincott Williams & Wilkins. The function of the lymphatic system is to (1) protect the body from infection and disease via the immune response and (2) to facilitate movement of fluid back and forth between the bloodstream and the interstitial spaces, removing excess fluid, blood waste, and protein molecules in the process of fluid exchange.3 In addition to the general chart review (see Chapter 2), it is important to gather the following information during examination of the patient with a suspected vascular disorder4–8: • Relevant medical history that includes diabetes mellitus, hypertension, hyperlipidemia, syncope or vertigo, and nonhealing ulcers. • Relevant social history that includes exercise and dietary habits, as well as the use of tobacco or alcohol. • History of recent prolonged bed rest and/or surgery or a long flight. • Pain in arms and legs. (Visceral pain and arthritis pain may radiate to the extremities.) • Presence of intermittent claudication (pain, ache, sense of fatigue, or other discomfort that occurs in the affected muscle group with exercise, particularly walking, and resolves with rest.) The speed, distance, and the site of the pain, including what relieves the pain, should be noted. • Buttock, hip, or thigh claudication typically occurs in patients with obstruction of the aorta and iliac arteries. Calf claudication characterizes femoral and popliteal artery stenosis. The gastrocnemius muscle consumes more oxygen during walking than other muscle groups in the leg and hence causes the most frequent symptom reported by patients. • Presence of nocturnal pain that can develop as the vascular occlusion worsens. This type of pain occurs when the patient is in bed and is caused by a combination of leg elevation and reduced cardiac output. • Presence of rest pain refers to pain that occurs in the absence of activity and with legs in a dependent position. Rest pain signals advanced occlusive disease, typically greater than 90% occlusion. • Presence or history of acute or chronic peripheral edema. If chronic, what is the patient’s baseline level of edema? • Precautions, such as weight bearing or blood pressure parameters after vascular surgery. Observation of the following features can help delineate the location and severity of vascular disease and help determine whether these manifestations are arterial or venous in origin1,4,5,9: • Skin color: Note the presence of any discoloration of the distal extremities/nail bed, which is indicative of decreased blood flow (e.g., mottled skin). • Hair distribution: Patchy hair loss on the lower leg may indicate arterial insufficiency. • Venous pattern: Dilation or varicosities—dilated, purplish, ropelike veins, particularly in the calf. • Edema or atrophy: Peripheral edema from right-sided congestive heart failure occurs bilaterally in dependent areas; edema from trauma, lymphatic obstruction, or chronic venous insufficiency is generally unilateral. Refer to Table 3-5 for grading of pitting edema. Measurement of the extremities may help to identify the edema or atrophy. With a flexible tape, measure: • The smallest possible circumference above the ankle • The largest circumference at the calf • The mid-thigh, a measured distance above the patella with the knee extended A difference greater than 1 cm just above the ankle or 2 cm at the calf is suggestive of edema. • Presence of petechiae: Small, purplish, hemorrhagic spots on the skin. • Skin lesions: Ulcers, blisters, or scars. • Digital clubbing: Could be indicative of poor arterial oxygenation or circulation. During the palpation portion of the examination, the physical therapist can assess the presence of pain and tenderness, strength and rate of peripheral pulses, respiratory rate, blood pressure, skin temperature, and limb girth (if edematous). Changes in heart rate, blood pressure, and respiratory rate may correspond to changes in the fluid volume status of the patient. For example, a decrease in fluid volume may result in a decreased blood pressure that results in a compensatory increase in heart and respiratory rates. The decreased fluid volume and resultant increased heart rate in this situation may then result in a decreased strength of the peripheral pulses on palpation. A decreased or absent pulse provides insight into the location of arterial stenoses.7 In patients with suspected or diagnosed peripheral vascular disease, monitoring distal pulses is more important than monitoring central pulses in the larger, more proximal vessels.4 The following system/scale is used to grade peripheral pulses10: Peripheral pulses can be assessed in the following arteries (see Chapter 3, Figure 3-6): • In patients who have disorders resulting in vascular compromise (e.g., diabetes mellitus, peripheral vascular disease, or hypertension), pulses should be monitored before, during, and after activity not only to determine any rate changes, but, more important, to determine any changes in the strength of the pulse. • Notation should be made if the strength of pulses is correlated to complaints of pain, numbness, or tingling of the extremity. • Compare the two extremities for color, temperature, and swelling. Bilateral coldness is most often due to cold environment and/or anxiety.5 • Carotid arteries should never be palpated simultaneously, as excessive carotid sinus massage can cause slowing of the pulse, cause a drop in blood pressure, and compromise blood flow to the brain. If the pulse is difficult to palpate, the patient’s head should be rotated to the side being examined to relax the sternocleidomastoid.10 Systemic blood pressure and the presence of bruits (whooshing sound indicative of turbulent blood flow from obstructions) are assessed through auscultation.4 Bruits are often indicative of accelerated blood flow velocity and flow disturbance at sites of stenosis.7 Bruits are typically assessed by physicians and nurses (see Chapter 3 for further details on blood pressure measurement). Various tests that can be performed clinically to evaluate vascular flow and integrity are described in Table 7-5. These tests can be performed easily at the patient’s bedside without the use of diagnostic equipment. The Wells Clinical Decision Rule for Deep Venous Thrombosis is described in Table 7-6. TABLE 7-5 *Variability is found in defining the time by different individuals, so capillary refill time should not be considered an observation with exquisite sensitivity and specificity and should be used more to confirm clinical judgment. †A 50% false-positive rate occurs with this test. Vascular laboratory studies are more sensitive. ‡ABI measurements may be of limited value in anyone with diabetes because calcification of the tibial and peroneal arteries may render them noncompressible. §An ABI of less than 0.95 is considered abnormal and is 95% sensitive for the angiographically verified peripheral arterial stenosis. Data from Seidel HM, Ball JW, Dains JE et al: Mosby’s guide to physical examination, ed 7, St Louis, 2010, Mosby; Lanzer P, Rosch J, editors: Vascular diagnostics: noninvasive and invasive techniques, peri-interventional evaluations, Berlin, 1994, Springer Verlag; Springhouse: Handbook of medical-surgical nursing, ed 4, Philadelphia, 2005, Lippincott Williams & Wilkins; Newberry L, Sheehy S, editors: Sheehy’s emergency nursing: principles and practice, ed 6, St Louis, 2009, Mosby; Dormandy JA, Rutherford RB: Management of peripheral arterial disease (PAD). TASC Working Group, J Vasc Surg 31:S1–S296, 2000; Hallet JW, Brewster DC, Darling RC, editors: Handbook of patient care in vascular surgery, ed 3, Boston, 1995, Little, Brown; Goodman CC: The hematologic system. In Goodman CC, Boisonnault WG, editors: Pathology: implications for the physical therapist, ed 3, Philadelphia, 2009, Saunders. TABLE 7-6 Wells Clinical Decision Rule (CDR) for Deep Venous Thrombosis *−2 to 0: Low probability of DVT (3%); 1 to 2: Moderate probability of DVT (17%); 3 or more: High probability of DVT (75%). Medical consultation is advised in the presence of low probability; medical referral is required with moderate or high score. From Wells PS, Anderson DR, Bormanis J et al: Value of assessment of pretest probability of deep-vein thrombosis in clinical management, Lancet 350(9094):1795-1798, 1997. Various noninvasive procedures can examine vascular flow. The phrases lower-extremity noninvasive studies and carotid noninvasive studies are general descriptions that are inclusive of the noninvasive tests described in Table 7-7. TABLE 7-7 Data from Black JM, Matassarin-Jacobs E, editors: Luckmann and Sorensen’s medical-surgical nursing: a psychophysiologic approach, ed 4, Philadelphia, 1993, Saunders, p 1286; Bryant RA, Nix DP: Acute and chronic wounds. Current management concepts, ed 4, St Louis, 2012, Mosby; Lanzer P, Rosch J, editors: Vascular diagnostics: noninvasive and invasive techniques, peri-interventional evaluations, Berlin, 1994, Springer Verlag; Kee JL, editor: Laboratory and diagnostic tests with nursing implications, ed 8, Stamford, CT, 2009, Appleton & Lange, p 606; Malarkey LM, Morrow ME, editors: Nurses manual of laboratory tests and diagnostic procedures, ed 2, Philadelphia, 2000, Saunders, p 359; Mettler FA: In Essentials of radiology, ed 2, Philadelphia, 2005, Saunders; Fahey VA, editor: Vascular nursing, ed 4, Philadelphia, 2003, Saunders; McCance KL, Huether SE, editors: Pathophysiology: the biologic basis for disease in adults and children, ed 6, St Louis, 2009, Mosby, p 1001; George-Gay B, Chernecky CC: In Clinical medical-surgical nursing: a decision-making reference, ed 1, Philadelphia, 2002, Saunders; Schroeder ML: Principles and practice of transfusion medicine, ed 10. In Lee GR, Foerster J, Lukens J et al, editors: Wintrobe’s clinical hematology, vol 1, Baltimore, 1999, Lippincott Williams & Wilkins, pp 817-874. The most common invasive vascular study is arteriography, typically referred to as contrast angiography (Figure 7-2). This study is performed by injecting radiopaque dye into the femoral, lumbar, brachial, or axillary arteries, followed by radiographic viewing. Blood flow dynamics, abnormal blood vessels, vascular anomalies, normal and abnormal vascular anatomy, and tumors are easily seen during the radiographic viewing. With the use of digital-subtraction angiography (DSA), bony structures can be obliterated from the picture. DSA is useful when adjacent bone inhibits visualization of the blood vessel to be evaluated.11 An angiogram is a picture produced by angiography. Angiography is generally performed before or during therapeutic interventions, such as percutaneous angioplasty, thrombolytic therapy, or surgical bypass grafting. FIGURE 7-2 Magnetic resonance angiography (MRA) of the aorta and lower extremity arterial circulation. (From Adam A: Grainger & Allison’s diagnostic radiology, ed 5, London, 2008, Churchill Livingstone.) Postangiogram care includes the following12: • Pressure dressings to the injection site with assessment for hematoma formation. • Intravenous fluid administration to help with dye excretion. Blood urea nitrogen (BUN) and creatinine are monitored to ensure proper renal function (refer to Chapter 9 for more information on BUN and creatinine). • Frequent vital sign monitoring with pulse assessments. • If a patient has been on heparin before angiography, the drug is not resumed for a minimum of 4 hours.12 The complications of arteriography can be due to the catheterization or due to the contrast agent that is injected (Table 7-8). TABLE 7-8 Complications of Contrast Arteriography From Belkin M, Owens CD, Whittemore AD et al: Peripheral arterial occlusive disease. In Townsend CM, Beauchamp RD, Evers BM et al, editors: Sabiston textbook of surgery: the biological basis of modern surgical practice, ed 18, Philadelphia, 2007, Saunders. In addition to the general chart review (see Chapter 2), the following questions are especially relevant in the evaluation of the patient with a suspected hematologic disorder13–15: • What are the presenting symptoms? • Was the onset of symptoms gradual, rapid, or associated with trauma or other disease? • Is the patient unable to complete daily activities secondary to fatigue? • Is there a patient or family history of anemia or other blood disorders, cancer, hemorrhage, or systemic infection? • Is there a history of blood transfusion? • Is there a history of chemotherapy, radiation therapy, or other drug therapy? • Has there been an environmental or occupational exposure to toxins? • Have there been night sweats, chills, or fever? • Is the patient easily bruised? During the hematologic evaluation, the patient is observed for the following13,16,17: • General appearance (for lethargy, malaise, or apathy) • Degree of pallor or flushing of the skin, mucous membranes, nail beds, and palmar creases. Pallor can be difficult to assess in dark-skinned individuals. In these individuals, lips, tongue, mucosa, and nail beds should be monitored. • Presence of petechiae (purplish, round, pinpoint, nonraised spots caused by intradermal or subcutaneous hemorrhage) or ecchymosis (bruising) The examination performed by the physician includes palpation of lymph nodes, liver, and spleen as part of a general physical examination. For specific complaints, the patient may receive more in-depth examination of a body system. Table 7-9 summarizes abnormal hematologic findings by body system on physical examination. TABLE 7-9 Signs and Symptoms of Hematologic Disorders by Body System Data from Black JM, Matassarin-Jacobs E, editors: Medical-surgical nursing: clinical management for continuity of care, ed 5, Philadelphia, 1997, Saunders. The physical therapist may specifically examine the following: • The presence, location, and severity of bone or joint pain using an appropriate pain scale (see Chapter 21) • Joint range of motion and integrity, including the presence of effusion or bony abnormality • Presence, location, and intensity of paresthesia • Blood pressure and heart rate for signs of hypovolemia (see Palpation in the Vascular Evaluation section for a description of vital sign changes with hypovolemia) The standard complete blood cell (CBC) count consists of a red blood cell (RBC) count, white blood cell (WBC) count, WBC differential, hematocrit (Hct) measurement, hemoglobin (Hgb) measurement, and platelet (Plt) count (Table 7-10). Figure 7-3 illustrates a common method used by the medical-surgical team to document portions of the CBC in progress notes. If a value is abnormal, it is usually circled within this “sawhorse” figure. TABLE 7-10 Complete Blood Cell Count: Values and Interpretation* *Lab values vary among laboratories. RBC, hemoglobin, and platelet values vary with age and gender. Data from Elin RJ: Laboratory reference intervals and values. In Goldman L, Bennett JC, editors: Cecil textbook of medicine, vol 2, ed 21, Philadelphia, 2000, Saunders, p 2305; Matassarin-Jacobs E: Assessment of clients with hematologic disorders. In Black JM, Matassarin-Jacobs E, editors: Medical-surgical nursing: clinical management for continuity of care, ed 5, Philadelphia, 1997, Saunders, p 1465; Mosby’s diagnostic and laboratory test reference, ed 8, St Louis, 2007, Mosby. FIGURE 7-3 Illustration of portions of the complete blood cell count in shorthand format. • The most important thing to consider when looking at hemoglobin values is the patient’s oxygen supply versus demand. Decreased Hgb levels can reduce oxygen transport capacity and subsequently reduce the oxygen supply, which can reduce a patient’s endurance level. • It is important to consider the trends in the Hgb and Hct levels. If Hct/Hgb levels are low at baseline, these patients may be able to tolerate activity. However, patients with acutely low levels of Hct/Hgb may or may not tolerate increased activity. • A physical therapist should be aware of signs and symptoms of hypoxia to major organs: brain, heart, and kidneys. • Monitoring of tolerance and modifications in the therapeutic plan may be indicated with low levels of Hct/Hgb.18 Hct is accurate in relation to fluid status; therefore Hct may be falsely high if the patient is dehydrated and falsely low if the patient is fluid overloaded.11 • Hct is approximately three times the Hgb value. • A low Hct may cause the patient to experience weakness, dyspnea, chills, or decreased activity tolerance, or it may exacerbate angina. • Patients with cancer such as leukemia or patients who are receiving cancer treatment will most likely present with lower Hct and Hgb values; therefore the therapist should proceed with caution in these patients. • The term pancytopenia refers to a significant decrease in RBCs, all types of WBCs, and platelets. • The term neutropenia refers to an abnormal decrease in WBCs, particularly neutrophils. • The term leukocytosis refers to an abnormal increase in circulating WBCs. • The term thrombocytopenia refers to a significant decrease in platelets. • The term thrombocytosis refers to an abnormal increase in platelets. RBC, Hct, and Hgb values are used to calculate three erythrocyte indices: (1) mean corpuscular volume (MCV), (2) mean corpuscular Hgb, and (3) mean corpuscular Hgb concentration (Table 7-11). At most institutions, these indices are included in the CBC. TABLE 7-11 Erythrocyte Indices: Values and Interpretation* Hct, Hematocrit; Hgb, hemoglobin; RBC, red blood cell. *Lab values vary among laboratories. Data from Elin RJ: Laboratory reference intervals and values. In Goldman L, Bennett JC, editors: Cecil textbook of medicine, vol 2, ed 21, Philadelphia, 2000, Saunders, p 2305; Matassarin-Jacobs E: Assessment of clients with hematologic disorders. In Black JM, Matassarin-Jacobs E, editors: Medical-surgical nursing: clinical management for continuity of care, ed 5, Philadelphia, 1997, Saunders, p 1466; and Pagana KD, Pagana TJ: Mosby’s diagnostic and laboratory test reference, ed 10, St Louis, 2011, Mosby, pp 830-833. The erythrocyte sedimentation rate (ESR), often referred to as the sed rate, is a measurement of how fast RBCs fall in a sample of anticoagulated blood. Normal values vary widely according to laboratory method. According to the Westergren method, the normal value for males is up to 15 mm per hour and the normal value for females is up to 20 mm per hour.11 The ESR is a reflection of acute-phase reaction in inflammation and infection. A limitation of the test is that it lacks sensitivity and specificity for disease processes. In addition, ESR cannot detect inflammation as quickly or as early as some other tests.19 ESR may be elevated in systemic infection, collagen vascular disease, and human immunodeficiency virus. It is a fairly reliable indicator of the course of disease. In general, as the disease worsens, the ESR increases; as the disease improves, the ESR decreases.11 ESR may be decreased in the presence of sickle cell disease, polycythemia, or liver disease or carcinoma. A blood sample may be examined microscopically for alterations in size and shape of the RBCs, WBCs, and platelets. RBCs are examined for size, shape, and Hgb distribution. WBCs are examined for proportion and the presence of immature cells. Finally, platelets are examined for number and shape.20 Peripheral blood smear results are correlated with the other laboratory tests to diagnose hematologic disease. An adjunct to the measurement of PT is the international normalized ratio (INR). The INR was created to ensure reliable and consistent measurement of coagulation levels among all laboratories. The INR is the ratio of the patient’s PT to the standard PT of the laboratory, raised by an exponent (the sensitivity index of the reagent) provided by the manufacturer.21 The PT, PTT, and INR are used in clinical conditions in which an increased risk of thrombosis is present—for example, treatment of deep venous thrombosis (DVT), thrombosis associated with prosthetic valves, and atrial fibrillation (Table 7-12).22 TABLE 7-12 DIC, Disseminated intravascular coagulation. *Values for PT and PTT vary between laboratories. Data from Pagana KD, Pagana TJ: Blood studies. In Mosby’s manual of diagnostic and laboratory tests, St Louis, 1998, Mosby; Mosby’s diagnostic and laboratory test reference, ed 8, St Louis, 2007, Mosby. The D-dimer assay provides (a highly specific) measurement of the amount of fibrin degradation. D-dimer tests have high sensitivity (95% to 99%) but are nonspecific (40% to 60%). Thrombotic problems such as DVT, pulmonary embolism (PE), and thrombosis of malignancy are associated with high levels of D-dimer. The test accurately identifies patients with DVT because its high sensitivity translates into a high negative predictive value. In other words, if the D-dimer test result is negative, the patient has a very low likelihood of having DVT. However, a positive D-dimer test result is less helpful because there are multiple conditions that may lead to elevated D-dimer titers, including advanced age, recent surgery, infection, inflammatory states and elevated liver enzyme levels. It is a simple and confirmatory test for disseminated intravascular coagulation (DIC). Levels of D-dimer can increase when a fibrin clot is lysed by thrombolytic therapy.11,23 Relevant history should include cancer and/or cancer treatment, trauma, and surgery, and onset of swelling at birth and/or puberty (primary lymphedema).3 Clinical evaluation should include a detailed description of skin integrity, use of body diagrams, both anterior-posterior (AP) and lateral to draw unusual body contours. This description should also include presence of edema or fibrosis on the trunk quadrants, the head, and the neck, as well as on the limbs, and the location and condition of scars, fibrotic area, and open wounds. Circumferential measurements accurately assess the shape and contour of a limb. Circumferential measurements should be taken at consistent locations/sites relative to the anatomical landmarks for reliable comparison between limbs and overtime. Volumetric measurement is a useful to measure the actual volume of the limb and is more helpful in cases of bilateral extremity edema, when no “normal” limb can be used for comparison. Both volumetric measurements and girth measurements have been shown to be reliable, but the two methods cannot be reliably interchanged.24 This section is divided into a discussion of vascular, hematologic, and lymphatic disorders. Vascular disorders are classified as arterial, venous, or combined arterial and venous disorders. Clinical findings differ between arterial and venous disorders, as described in Table 7-13. TABLE 7-13 Comparison of Clinical Findings of Arterial and Venous Disorders Data from Black JM, Matassarin-Jacobs E, editors: Luckmann and Sorensen’s medical-surgical nursing: a psychophysiologic approach, ed 4, Philadelphia, 1993, Saunders, p 1261. Atherosclerosis is a diffuse and slowly progressive process characterized by areas of hemorrhage and the cellular proliferation of monocytes, smooth muscle, connective tissue, and lipids. The development of atherosclerosis begins early in life. In addition to the risk factors listed in Box 7-1, a high level of an inflammatory biomarker, C-reactive protein, has been identified as a good predictive marker for early identification of atherosclerosis.25 Waist circumference and weight gain are the strongest predictors of early atherosclerosis in healthy adults.26 Atherosclerosis is the underlying cause of approximately 90% of all myocardial infarction and a large proportion of strokes and ischemic gangrenes.27 Clinical manifestations of atherosclerosis result from decreased blood flow through the stenotic areas. Signs and symptoms vary according to the area, size, and location of the lesion, along with the age and physiologic status of the patient. As blood flows through a stenotic area, turbulence will occur beyond the stenosis, resulting in decreased blood perfusion past the area of atherosclerosis. Generally, a 50% to 60% reduction in blood flow is necessary for patients to present with symptoms (e.g., pain). Turbulence is increased when there is an increase in blood flow to an area of the body, such as the lower extremities during exercise. When atherosclerosis develops slowly, collateral circulation develops to meet the needs.24 A patient with no complaint of pain at rest may therefore experience leg pain (intermittent claudication [IC]) during walking or exercise as a result of decreased blood flow and the accumulation of metabolic waste (e.g., lactic acid).4,28,29 The following are general signs and symptoms of atherosclerosis30: • Peripheral pulses that are slightly reduced to absent. • Presence of bruits on auscultation of major arteries (i.e., carotid, abdominal aorta, iliac, and femoral). • Coolness and pallor of skin, especially with elevation. • Presence of ulcerations, atrophic nails, and hair loss. • Subjective reports of continuous burning pain in the lower extremities at rest that is aggravated with elevation (ischemic pain) and relieved with placing the leg over the edge of the bed.24 Pain at rest is usually indicative of severe (80% to 90%) arterial occlusion. • Subjective reports of calf or lower-extremity pain, ache or cramp induced by walking (intermittent claudication) and relieved by rest. Symptoms similar to intermittent claudication may have a neurologic origin from lumbar canal stenosis or disc disease. These symptoms are referred to as pseudoclaudication or neurologic claudication. Table 7-14 outlines the differences between true claudication and pseudoclaudication.31 TABLE 7-14 Differentiating True Intermittent Claudication from Pseudoclaudication Data from Young JR, Graor RA, Olin JW et al, editors: Peripheral vascular diseases, St Louis, 1991, Mosby, p 183; Fritz JM: Spinal stenosis. In Placzek JD, Boyce DA, editors: Orthopaedic physical therapy secrets, Philadelphia, 2001, Hanley & Belfus, p 344. All patients with claudication should be strongly advised to stop smoking. The beneficial effect of exercise training in patients with IC is well proven. The improvement in walking distance has been reported to be between 30% and 200%. A specific exercise program gives a more marked improvement than if the patient tries to exercise on his or her own. The greatest improvement in walking distance until pain develops seems to occur with an exercise duration of longer than 30 minutes per session and a frequency of at least three sessions per week. Walking should be used as a mode of exercise, and it should be performed at nearly maximum pain. The program should last at least 6 months.27 Medications that have been used in managing intermittent claudication include pentoxifylline and cilostazol.32 Treatment of atherosclerotic disease is based on clinical presentation and can range from risk-factor modifications (e.g., low-fat diet, increased exercise, and smoking cessation) to pharmacologic therapy (e.g., anticoagulation and thrombolytics) to surgical resection and grafting. Modification of risk factors has been shown to be the most effective method to lower the risk of morbidity (heart attack or stroke) from atherosclerosis.33,34 An aneurysm is a localized dilatation or outpouching of the vessel wall that results from degeneration and weakening of the supportive network of protein fibers with a concomitant loss of medial smooth muscle cells. Aneurysms most commonly occur in the abdominal aorta or iliac arteries, followed by the popliteal, femoral, and carotid vessels.28,33,35,36 The exact mechanism of aneurysm formation is not fully understood but includes a combination of the following: • Genetic abnormality in collagen (e.g., with Marfan’s syndrome) • Aging and natural degeneration of elastin • Increased proteolytic enzyme activity A true aneurysm is defined as a 50% increase in the normal diameter of the vessel36 and involves weakening of all three layers of the arterial wall. True aneurysms are also generally fusiform and circumferential in nature. False and saccular aneurysms are the result of trauma from dissection (weakness or separation of the vascular layers) or clot formation (Figure 7-4). They primarily affect the adventitial layer.35 FIGURE 7-4 True and false aneurysms. Center, Normal vessel. Left, True aneurysm. The wall bulges outward and may be attenuated but is intact. Right, False aneurysm. The wall is ruptured, and there is a collection of blood (hematoma) that is bounded externally by adherent extravascular tissues. (From Kumar V: Robbins and Cotran pathologic basis of disease, ed 8, Philadelphia, 2010, Saunders.) Abdominal aortic aneurysm is dilatation of the abdominal aorta to more than 3 cm in diameter. These aneurysms can be infrarenal, juxtarenal or suprarenal, according to the relationship to the renal arteries.27 Approximately 80% of the aneurysms are identified incidentally on abdominal ultrasound, computed tomography (CT) scan, magnetic resonance imaging (MRI), or plain x-ray.37 Aneurysms will rupture if the intraluminal pressure exceeds the tensile strength of the arterial wall. Rupture is mostly likely to occur in aneurysms that are 5 cm or larger.24 The following are additional clinical manifestations of aneurysms: • Popliteal aneurysm presents as a pulsating mass, 2 cm or more in diameter. Femoral aneurysms presents as a pulsating mass in the femoral area on one or both sides.24 In thin individuals, an aortic aneurysm may be seen as a pulsating swelling in the upper abdomen.27 • Ischemic manifestations (described earlier in the Atherosclerosis section), if the aneurysm impedes blood flow. Most abdominal aneurysms are asymptomatic, but intermittent or constant pain in the form of mild to severe mid-abdominal or lower back discomfort is present in some form in 25% to 30% of cases. Groin or flank pain may be experienced because of increasing pressure on other strutures.24 Most of the aneurysms are relatively asymptomatic until an embolus dislodges from the aneurysm or the aneurysm ruptures.36 • Cerebral aneurysms, commonly found in the circle of Willis, present with increased intracranial pressure and its sequelae (see Chapter 6 for more information on intracranial pressure).35 • Aneurysms that result in subarachnoid hemorrhage are also discussed in Chapter 6. • Low back pain (aortic aneurysms can refer pain to the low back). • Dysphagia (difficulty swallowing) and dyspnea (breathlessness) resulting from the enlarged vessel’s compressing adjacent organs. Surgical resection and graft replacement are generally the desired treatments for aneurysms.38 However, endovascular repair of abdominal aneurysms is demonstrating favorable results. Endovascular repair involves threading an endoprosthesis through the femoral artery to the site of the aneurysm. The endoprosthesis is then attached to the aorta, proximal to the site of the aneurysm, and distal to the iliac arteries. This effectively excludes the aneurysm from the circulation, which minimizes the risk of rupture.36 Non–surgical candidates must have blood pressure and anticoagulation management.38 Aortic dissection is caused by an intimal tear, which allows creation of a false lumen between the media and adventitia. A history of Marfan’s syndrome or hypertension is usually present.39 Aortic dissection occurs at least twice as frequently in men than in women.7 Signs and symptoms generally reflect the type of aortic dissection (whether type A or type B [Figure 7-5]) and the extent of cardiovascular involvement.40 Signs and symptoms of aortic dissection include40: FIGURE 7-5 Classification of aortic dissections. A, Dissection of ascending aorta (type A). B, Dissection of descending aorta (type B). (From Kumar V: Robbins and Cotran pathologic basis of disease, ed 8, Philadelphia, 2010, Saunders.) • Pain: Sudden and excruciating pain in the chest (90% of the patients) or the upper back is the most common initial symptom. Another important characteristic of the pain is its tendency to migrate to the neck, abdomen, or groin, generally following the path of dissection. • Shock: Cardiogenic or hypovolemic shock may be secondary to cardiac tamponade from aortic rupture into the pericardium, dissection or compression of the coronary arteries, acute aortic regurgitation, or acute blood loss. • Hypertension: More than 50% of patients with distal dissection are hypertensive, and severe hypertension with diastolic pressure as high as 160 mm Hg may be encountered with distal dissection. Severe hypertension may be due to renal ischemia. • Murmur of aortic regurgitation. This may be present in 50% of the patients with proximal dissection and may occur because of widening of the aortic annulus or actual disruption of the aortic valve leaflets. • Pleural effusions: Pleural effusion, which occur most frequently in the left chest, can be caused by the rupture of the dissection into the pleural space or by weeping of fluid from the aorta as the result of an inflammatory reaction to the dissection.41 • Neurological manifestations (cerebrovascular accident and, rarely, altered consciousness, coma). The chest radiograph may be the first clue to the diagnosis of aortic dissection, but the findings on the chest radiograph are nonspecific, subject to interobserver variability and in many cases completely normal.7 Electrocardiogram (ECG) findings in these patients are nonspecific.7,39 Transesophageal echocardiography (TEE) and CT scan are the primary diagnostic tests used by most institutions to diagnose aortic dissection.42 TEE is highly accurate for the evaluation and diagnosis of acute aortic dissection, with sensitivity (98%) and specificity (94% to 97%). Contrast CT is also highly accurate for diagnosing aortic dissection, with sensitivity and specificity of 95% to 98%. MRI is a highly accurate noninvasive technique for evaluating aortic dissection but is rarely used as the initial test for diagnostic evaluation of acute dissection. MRI is known to have high sensitivity (95% to 100%) and specificity (94% to 98%) for the detection of aortic dissection.7 Arterial thrombosis occurs in areas of low or stagnant blood flow, such as atherosclerotic or aneurysmal areas. The reduced or turbulent blood flow in these areas leads to platelet adhesion and aggregation, which then activates the coagulation cycle to form a mature thrombus (clot). Blood flow may then be impeded, potentially leading to tissue ischemia with subsequent clinical manifestations.35,38 An arterial embolus is a fragment of thrombus, fat, atherosclerotic plaque, bacterial vegetation, or air that mobilizes within the arterial vessels and obstructs flow distal to the embolus.43 Arterial emboli arise from areas of stagnant or disturbed blood flow in the heart or aorta. Acute arterial embolus is a surgical emergency. The likelihood of limb salvage decreases after 4 to 6 hours.42 The most common sources of arterial emboli are listed in Table 7-15. TABLE 7-15 From Belkin M, Owens CD, Whittemore AD et al: Peripheral arterial occlusive disease. In Townsend CM, Beauchamp RD, Evers BM et al, editors: Sabiston textbook of surgery: the biological basis of modern surgical practice, ed 18, Philadelphia, 2007, Saunders. Areas in which arterial emboli tend to lodge and interrupt blood flow are arterial bifurcations and areas narrowed by atherosclerosis (especially in the cerebral, mesenteric, renal, and coronary arteries). Signs and symptoms of thrombi, emboli, or both depend on the size of the occlusion, the organ distal to the clot, and the collateral circulation available.35 When arterial thrombosis or embolism is suspected, the affected limb must be protected by proper positioning below the horizontal plane, and protective skin care must be provided. Heat or cold application and massage are to be avoided.24 Treatment of thrombi, emboli, or both includes anticoagulation with or without surgical resection of the atherosclerotic area that is predisposing the formation of thrombi, emboli, or both. Medical management of arterial thrombosis can also include antithrombotic drugs (e.g., tissue factor or factor Xa inhibitors) or combined antithrombotic therapy with aspirin, a thienopyridine and warfarin, or both.44 Hypertension is an elevated arterial blood pressure, both systolic and diastolic, that is abnormally sustained at rest (Table 7-16). TABLE 7-16 Hypertension as It Relates to Different Age Groups Data from Bullock B: Pathophysiology: adaptations and alterations in function, ed 4, Philadelphia, 1996, Lippincott-Raven, p 517; Chobanian A, et al: The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. The JNC 7 report, Hypertension 2003, 42:1206-1252, 2003.
Vascular System and Hematology
Preferred Practice Patterns
Body Structure
Vascular System Structure
Layer
Description
Function
Tunica intima
Innermost layer: Endothelial layer over a basement membrane
Provides a smooth surface for laminar blood flow
Tunica media
Middle layer: Smooth muscle cells and elastic connective tissue with sympathetic innervation
Constricts and dilates for blood pressure regulation
Tunica adventitia
Outermost layer: Composed of collagen fibers, lymph vessels, and the blood vessels that supply nutrients to the blood vessel
Protects and attaches blood vessels to nearby structures
Hematologic System Structure
Lymphatic System Structure
Body Function
Function
Method
Oxygen and carbon dioxide transport
Binding to hemoglobin; dissolved in plasma
Nutrient and metabolite transport
Bound to plasma proteins; dissolved in plasma
Hormone transport
In plasma
Transport of waste products to kidneys and liver
In plasma
Transport of cells and substances involved in immune reactions
In plasma to site of infection or foreign body
Clotting at breaks in blood vessels
Hemostasis
Maintenance of fluid balance
Blood volume regulation
Body temperature regulation
Peripheral vasoconstriction or dilation
Maintenance of acid-base balance
Acid-base regulation
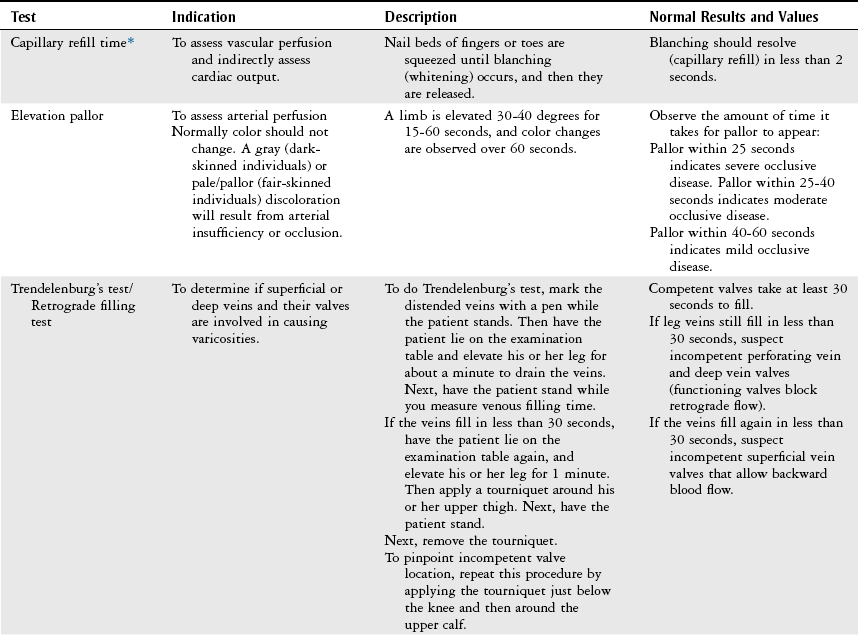
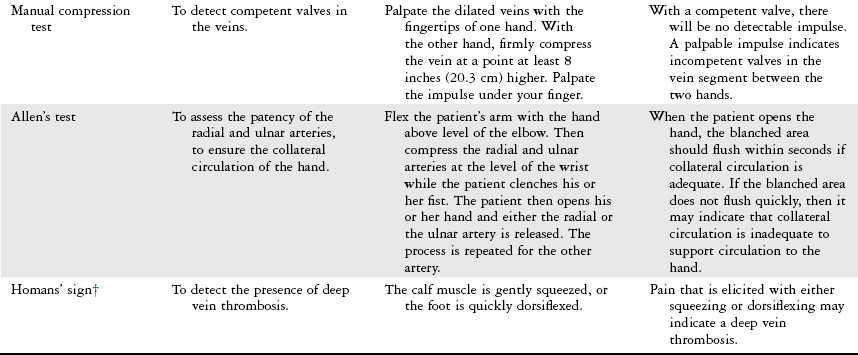
Physical Examination
Vascular Evaluation
History
Inspection
Palpation
Physical Therapy Considerations
Auscultation
Vascular Tests

Clinical Characteristic
Score*
Active cancer (treatment ongoing within previous 6 mo or palliative)
1
Paralysis, paresis, or recent plaster immobilization of the lower extremities
1
Recently bedridden for more than 3 days or major surgery, within 4 weeks
1
Localized tenderness along the distribution of the deep venous system
1
Entire leg swollen
1
Calf swelling by more than 3 cm when compared with the asymptomatic leg (measured 10 cm below tibial tuberosity)
1
Pitting edema (greater in the symptomatic leg)
1
Collateral superficial veins (nonvaricose)
1
Alternative diagnosis as likely as or more likely than deep vein thrombosis
−2
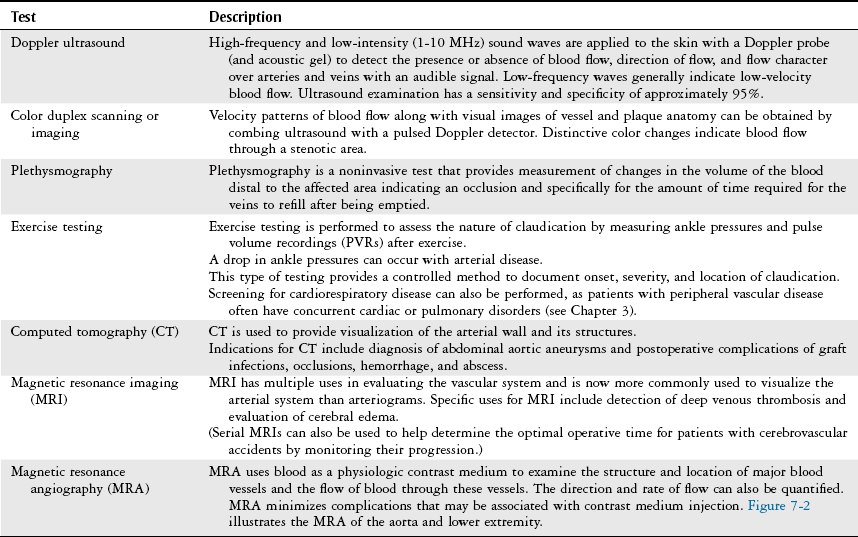
Diagnostic Studies
Noninvasive Laboratory Studies.
Invasive Vascular Studies.
Hematologic Evaluation
History
Inspection
Palpation
Laboratory Studies
Complete Blood Cell Count.
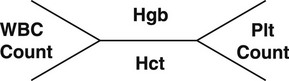
Hct, Hematocrit; Hgb, hemoglobin; Plt, platelet; WBC, white blood cell.
Physical Therapy Considerations
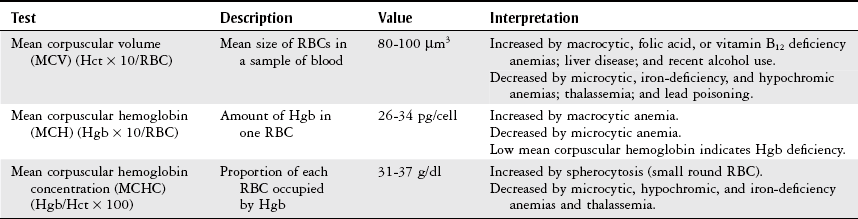
Erythrocyte Indices.
Erythrocyte Sedimentation Rate.
Peripheral Blood Smear.
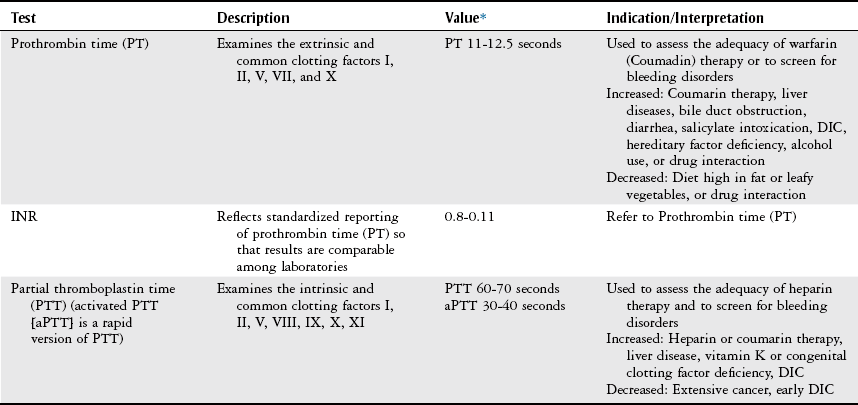
Coagulation Profile.
D-Dimer.
Lymphatic Evaluation
Health Conditions
Vascular Disorders
Clinical Finding
Arterial Disorders
Venous Disorders
Edema
May or may not be present
Present
Worse at the end of the day
Improves with elevation
Muscle mass
Reduced
Unaffected
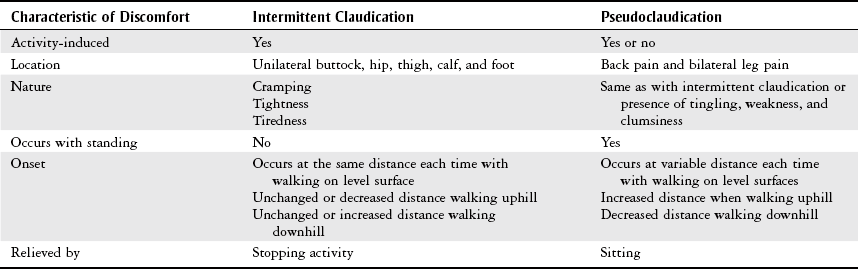
Pain
Intermittent claudication
Cramping
Worse with elevation
Aching pain
Exercise improves pain
Better with elevation
Cramping at night
Paresthesias, pruritus (severe itching)
Leg heaviness, especially at end of day
Pulses
Decreased to absent
Possible systolic bruit
Usually unaffected, but may be difficult to palpate if edema is present
Skin
Absence of hair
Small, painful ulcers on pressure points, especially lateral malleolus
Normal toenails
Tight, shiny skin
Thickened toenails
Broad, shallow, painless ulcers of the ankle and lower leg
Color
Pale
Dependent cyanosis
Brown discoloration
Dependent cyanosis
Temperature
Cool
May be warm in presence of thrombophlebitis
Sensation
Decreased light touch
Occasional itching, tingling, and numbness
Pruritus
Arterial Disorders
Atherosclerosis.
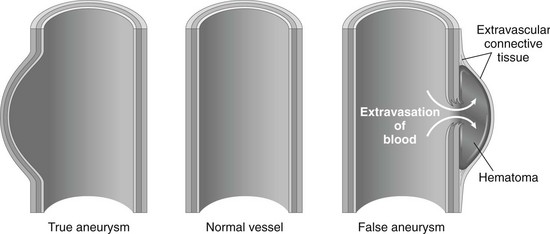
Aneurysm.
Physical Therapy Considerations.
Aortic Dissection.
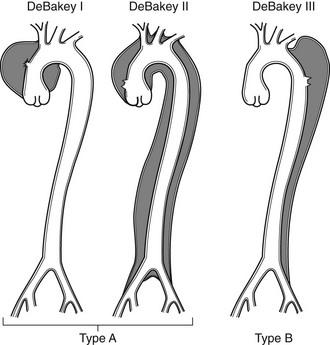
Arterial Thrombosis.
Arterial Emboli.
Source
Percentage
Cardiac
80%
Atrial fibrillation
50%
Myocardial infarction
25%
Other
5%
Noncardiac
10%
Aneurysmal disease
6%
Proximal artery
3%
Paradoxical emboli
1%
Other or idiopathic
10%
Hypertension.
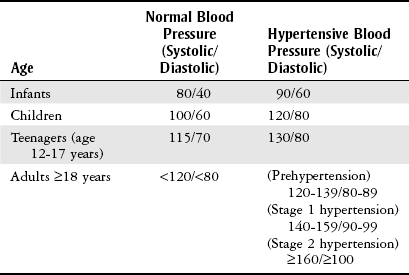
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Vascular System and Hematology
Only gold members can continue reading. Log In or Register to continue