Abstract
Objective
To validate a French version of the Roland–Morris Disability Questionnaire (RMDQ) in patients with chronic low back pain (LBP).
Material and methods
Fifty-eight patients due to participate in a functional rehabilitation programme for chronic low back pain were included prospectively. The RMDQ, the Quebec Back Pain Disability Scale (QBPDS) and the “daily activities” subscale of the Dallas Pain Questionnaire (DPQ) were administered. The RMDQ was assessed for internal consistency, reliability, criterion validity and sensitivity to change.
Results
Cronbach’s alpha for the RMDQ was 0.84. The intraclass correlation coefficient [95% confidence interval] was 0.89 [0.83–0.93]. The RMDQ score was correlated with the QBPDS score ( r = 0.713) and the DPQ’s “daily activities” subscore ( r = 0.514). The results of assessments before and after functional rehabilitation showed that the French version of the RMDQ had very high sensitivity to change (effect size: 1.49) and was more sensitive than the QBPDS and the DPQ’s “daily activities” subscore.
Conclusions
Our study validated the French version of the RMDQ in patients suffering from chronic low back pain. Furthermore, we highlighted the questionnaire’s very high sensitivity to change.
Résumé
Objectif
L’objectif de cette étude était de valider le questionnaire Roland-Morris dans sa version française, dans l’indication des lombalgies chroniques.
Patients et méthodes
Cinquante-huit patients lombalgiques chroniques candidats à un programme de restauration fonctionnelle ont été inclus de façon prospective. Les questionnaires Roland-Morris, Québec et Dallas – domaine « activités quotidiennes » – ont été appliqués. La validation du questionnaire Roland-Morris a compris l’étude de sa cohérence interne, de sa reproductibilité, de sa validité contre critère et de sa sensibilité au changement.
Résultats
Le coefficient alpha de Cronbach du questionnaire Roland-Moris était de 0,84. Le coefficient de corrélation intraclasse était de 0,89 (IC95 % 0,83–0,93). Le score Roland-Morris était corrélé au score de Québec ( r = 0,713) et du domaine « activités quotidiennes » du questionnaire de Dallas ( r = 0,514). Les résultats des évaluations avant et après restauration fonctionnelle attestaient d’une très bonne sensibilité au changement du questionnaire Roland-Morris (taille de l’effet 1,49). Celle-ci apparaissait supérieure à celle du score de Québec et à celle du score du domaine « activités quotidiennes » du questionnaire de Dallas.
Conclusions
Notre travail a permis la validation du questionnaire Roland-Morris dans sa version française au cours des lombalgies chroniques. Nous révélons également une sensibilité au changement particulièrement performante.
1
English version
1.1
Introduction
Low back pain (LBP) is common in the general adult population in Europe, North America and Australia . This condition leads to marked limitations of activity and affects the level of handicap experienced by the patient . With the recognition that chronicity has several determinants, multidisciplinary management of patients with persistent, invalidating LBP has now been widely adopted . The first step in this care programme consists of a clinical, functional and psychosocial evaluation. There are many tools available for this purpose and French versions of some of these have been validated in the indication of chronic LBP. This is the case for the Quebec Back Pain Disability Scale (QBPDS), the Dallas Pain Questionnaire (DPQ) and the Fear-Avoidance Beliefs Questionnaire . In contrast, a French version of the Roland–Morris Disability Questionnaire (the RMDQ, which is widely used and recommended in the management of chronic LBP) has only been validated in the indication of acute LBP (as the Échelle d’Incapacité Fonctionnelle pour l’Évaluation des Lombalgies questionnaire) . The objective of the present study was thus to validate a French version of the RMDQ in the indication of chronic LBP. Given the context in which the use of functional scales is recommended, it seemed logical to target a population of LBP patients due to take part in a multidisciplinary functional rehabilitation programme and who would thus meet the inclusion criteria for this type of care.
1.2
Patients and methods
1.2.1
Patients
Chronic LBP patients having volunteered for a functional rehabilitation programme were included prospectively. The inclusion criteria were as follows:
- •
age 18 or over;
- •
non specific LBP for at least 3 months;
- •
failure of pharmacological or surgical treatments when they were indicated;
- •
ability to read French;
- •
the provision of written, informed consent to participation in both the study and the functional rehabilitation programme.
The main non-inclusion criteria were heart or respiratory failure and signs of myocardial ischemia on the electrocardiogram recorded during a pretreatment test.
1.2.2
The functional rehabilitation programme
The five-days-a-week, four-week functional rehabilitation programme was performed in a residential rehabilitation centre. The programme was led by a specialist in physical and rehabilitation medicine, a psychiatrist, physiotherapists, occupational therapists, nurses and nursing assistants. The physical exercise programme included:
- •
trunk and lower limb muscle stretching sessions;
- •
trunk and limb muscle strength training;
- •
aerobic exercise (indoors on an exercise bike;
- •
a rowing machine and a treadmill but also outdoors);
- •
lifting/carrying exercises.
The patients’ progress (in terms of muscle strength, lifting/carrying and aerobic activities) was determined by pre-set ladders. The criteria for progression were the number of reps per set, load, power and duration.
1.2.3
Validation
The patients were evaluated seven days before the start of the functional rehabilitation programme (D−7), at the start (D0) and at the end (D25). The evaluation criteria were the RMDQ , the QBPDS and the DPQ’s “daily activities” subscale . As with the RMDQ, the QBPDS and the DPQ’s “daily activities” subscale both investigate the functional impact of non specific LBP. The 24-item RMDQ grades functional impairment from 0 (no impairment) to 24 (the worse possible impairment) . The QBPDS comprises 20 items and is scored from 0 (no impairment) to 100 (the worse possible impairment) . The DPQ’s “daily activities” subscale contains 7 items and is also scored from 0 (no impairment) to 100 (the worse possible impairment) .
The study duration was determined as a function of the required sample size. The French Rheumatology Society’s Groupe d’Étude des Lombalgies back pain working group has validated a French version of the DPQ in a 54-patient study . This number was increased slightly for validation of the RMDQ, in order to take account of possible study withdrawals and missing data. Hence, the sample size was set to 60 patients on the basis of the inclusion criteria and the treatment of five patients a month for 12 months. The study data for the population as a whole were analyzed in a single batch at the end of the study inclusion and treatment periods. Our validation of the French version of the RMDQ included an assessment of its internal coherence, reproducibility, criterion validity and sensitivity to change.
1.2.4
Internal coherence
The RMDQ administered at D0 was used to study internal coherence (as measured by Cronbach’s alpha coefficient) . A value above 0.60 was considered to be acceptable.
1.2.5
Reproducibility
The RMDQs administered at D−7 and D0 were used to assess reproducibility (according to the Bland–Altman method and the intraclass correlation coefficient [ICC] and 95% confidence interval [CI]). An ICC above 0.60 was considered to attest to good reproducibility .
1.2.6
Criterion validity
The RMDQ, QBPDS and DPQ administered at D0 were used to assess criterion validity according to Spearman’s coefficient . Coefficients above 0.5 and 0.7 were considered to attest to moderate and good correlations, respectively.
1.2.7
Sensitivity to change
The RMDQ, QBPDS and DPQ administered at D0 and D25 were used to evaluate sensitivity to change in the population as a whole (using a Wilcoxon test with a value P < 0.05 and based on the observed effect size, i.e. the difference between the D0 and D25 values divided by the initial standard deviation). Values of 0.2, 0.5 and 0.8 respectively indicated low, moderate and large sensitivity . The percentage change in patients reporting an improvement was also compared with that in patients not reporting an improvement (in a Mann–Whitney test).
1.3
Results
Fifty-eight consecutive patients were included ( Table 1 ). Six of these were in the rehabilitation centre at the start of the functional rehabilitation programme and were not evaluated at D−7. Five patients (including two of the six patients just mentioned) had not filled out the RMDQ at D25.
| Number | 58 |
| Age in years, mean ± SD | 42.9 ± 7.7 |
| Female/male gender ratio, n (%) | 20 (34.5)/38 (65.5) |
| Months since pain onset, mean ± SD (range) | 55.9 ± 88.3 (4–456) |
| Back operations, n (%) | 22 (37.9) |
| Work invalidity, n (%) | 32 (55.2) |
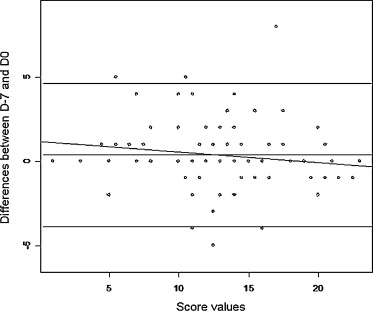
Cronbach’s alpha coefficient was 0.84; this was well above the threshold of 0.60 and thus attested to very good internal coherence for the RMDQ in the population studied here. The ICC [95% CI] was 0.89 [0.83–0.93] and thus indicated good reproducibility. The latter was confirmed by a Bland–Altman plot showing that the difference between D−7 and D0 was close to zero (regardless of the score’s value) ( Fig. 1 ).
The RMDQ score was correlated with the QBPDS score ( r = 0.713) and the DPQ’s “daily activities” score ( r = 0.514, P < 0.0001). According to our preselected thresholds, these correlations were respectively good and moderate. The results of the evaluations before and after functional rehabilitation are given in Table 2 and demonstrate very high sensitivity to change – greater than that of the QBPDS score or that of the DPQ’s “daily activities” subscore. Moreover, the patients reporting an improvement ( n = 42) had greater mean change in the RMDQ score (−39 ± 41 [range:−100 to 100]) than those not reporting an improvement (−12 ± 17 [range: −39 to 25; P = 0.013]).
| Scales | n | Mean ± SD at D0 | Mean ± SD at D25 | Effect size | P value |
|---|---|---|---|---|---|
| Quebec Back Pain Disability Scale | 52 | 38.7 (16.1) | 29.5 (19.5) | 0.57 | 0.0003 |
| Dallas Pain Questionnaire – “daily activities” subscale | 51 | 63.1 (13.5) | 51.1 (21) | 0.89 | 0.0006 |
| Roland–Morris Disability Questionnaire | 53 | 15.7 (4.9) | 8.3 (6.3) | 1.49 | 0.0001 |
1.4
Discussion
This work provided new information on the metrological properties of a French version of the RMDQ in a population of LBP patients. Under our conditions, the questionnaire had very good internal coherence, good reproducibility and good convergent validity and was highly sensitive to change. Indeed, its sensitivity to change appears to be greater than that of the QBPDS and the DPQ’s “daily activities” score. Our results thus validate the French version of the RMDQ in chronic LBP.
The French version used in the present study was obtained through careful translation and adaptation of the English-language original . Although this French version had already been validated in a population of patients suffering from acute LBP, we expected its metrological properties to differ when administered to patients with chronic LBP. However, the results were similar to those observed in acute LBP. In the latter patient population, the questionnaire’s reproducibility (according to the ICC) was 0.89 and there was a statistically significant change in the score after one week of follow-up. Our results are also similar to those obtained with the original English version of the RMDQ . Cronbach’s alpha ranged from 0.84 to 0.96 and the reproducibility in LBP patients (as evaluated by the Spearman coefficient) was 0.72. Good correlations with the QBPDS score have also been described and the original RMDQ’s sensitivity to change is well documented. In contrast to our present observations, the English version of the RMDQ did not appear to be more sensitive than the QBPDS scale. This disparity could be due to interstudy differences in populations, time intervals between evaluations, initial scores and treatments applied.
The validation process that we followed here complied with the recommended method . The prospective nature of our study, our characterization of the population and our prior definition of thresholds for internal coherence, reproducibility, correlations and sensitivity to change contributed to the robustness of our results. The agreement with previous studies of the original version of the RMDQ and its French version also suggests that our findings are robust. Moreover, the change in the RMDQ score observed here agrees with the outcomes of functional rehabilitation programmes for chronic LBP .
The RMDQ is thus the second English-language functional scale (after the QBPDS) to have been validated in French for chronic LBP . Unsurprisingly, we found that the two scales converged. With 24 and 20 items respectively, the questionnaires’ acceptabilities (although not strictly evaluated in the present study) appeared to be similar. On one hand, the six-point Likert scale used for each QBPDS item is more elaborate than direct selection of the statements in the RMDQ but is nevertheless simple. On the other hand, the RMDQ’s response mode means that some aspects of the functional handicap associated with LBP may not be addressed. It has been suggested that a binary “yes”/“no” response system would circumvent this problem . However, our study highlighted an important difference between the two questionnaires. The effect size observed for the RMDQ was more than twice that observed for the QBPDS. On the basis of the selected thresholds here, the effect size for the former scale was qualified as “large”, whereas that of the second corresponds to “medium”. This information should be considered when choosing the efficacy criterion in a therapeutic trial intended to evaluate functional impact during chronic LBP. It should also be considered in the analysis and interpretation of the results of clinical studies in this same indication.
Our validation work had a number of limitations. Firstly, our study design did not incorporate an assessment of the RMDQ’s acceptability. It nevertheless appears that all the patients seen at D−7 and D0 had filled out the questionnaire. Four of the five missing datasets at D25 corresponded to patients having prematurely withdrawn from the functional rehabilitation programme and were thus unrelated to the questionnaire. Administration of the French version of the RMDQ was qualified as “quick” when applied to acute LBP . The acceptability of the English version is well established . The content validity was not studied either. In fact, the RMDQ was elaborated more than 30 years ago and the items’ relevance for patients was tested in a qualitative approach. Society has changed significantly since the publication of this questionnaire and it may be the case that some items are no longer appropriate. Conversely, notions not tested in the questionnaire may have become significant for today’s patients. Thirdly, the sample size ( n = 58) could be qualified as small. The guidelines on the number of patients to be included in validation studies are contradictory . Our sample size of our population met the criteria stated in some guidelines but not in others and was similar to or greater than those studied when validating the French versions of the DPQ and QBPDS . Lastly, our population was not representative of LBP patients as a whole, since the inclusion criterion was LBP for 3 months or more. Our patients had a severe functional handicap that justified intensive, multidisciplinary care. This aspect is attested to by the particularly long duration of LBP in our study population.
In conclusion, we validated a French version of the RMDQ in patients suffering from chronic LBP. Previously, this version had only been validated in patients with on-going, acute LBP. We observed a particularly high sensitivity to change. Our results provide new, useful data on a questionnaire that is widely recommended and applied in chronic LBP.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
2
Version française
2.1
Introduction
Les lombalgies communes sont fréquentes dans la population générale adulte en Europe, sur le continent nord américain et en Australie . Elles peuvent être à l’origine d’une restriction notable d’activité, conditionnant le handicap perçu par le patient en raison de sa maladie . La prise en charge multidisciplinaire des lombalgies persistantes et invalidantes est maintenant un principe admis . Elle répond au déterminisme multifactoriel de la condition de chronicité. La première étape de cette prise en charge comprend une évaluation clinique, fonctionnelle et psychosociale. Les outils à disposition pour cette évaluation sont nombreux . Un certain nombre d’entre eux ont fait l’objet d’une validation dans l’indication des lombalgies chroniques en versions françaises. C’est le cas de l’indice fonctionnel de Québec, de l’échelle de qualité de vie de Dallas et du questionnaire de peurs et croyances inadaptées, FABQ . En revanche, le questionnaire Roland-Morris, utilisé et recommandé dans la prise en charge des lombalgies chroniques n’a été validé dans sa version française, échelle d’incapacité fonctionnelle pour l’évaluation des lombalgies (EIFEL), qu’au cours des lombalgies aiguës . L’objectif de cette étude est par conséquent la validation de la version française du questionnaire Roland-Morris dans l’indication des lombalgies chroniques. Compte tenu du cadre d’utilisation recommandé des échelles fonctionnelles, il est apparu logique de cibler une population de patients lombalgiques chroniques candidat à un programme multidisciplinaire de restauration fonctionnelle et par-là même répondant aux critères d’inclusion pour ce type de prise en charge.
2.2
Patients et méthodes
2.2.1
Patients
Des patients candidats à un programme de restauration fonctionnelle pour lombalgie chronique ont été inclus de façon prospective. Les critères d’inclusion étaient les suivants :
- •
âge > 17 ans ;
- •
lombalgie commune depuis au moins 3 mois ;
- •
échec des traitements médicaux ou chirurgicaux lorsqu’ils étaient indiqués ;
- •
lecture du français ;
- •
consentement éclairé écrit pour l’évaluation et le suivi du programme de restauration fonctionnelle.
Les critères de non inclusion étaient une insuffisance cardiaque ou respiratoire et des signes d’ischémie myocardique sur l’ECG d’effort préthérapeutique systématique.
2.2.2
Programme de restauration fonctionnelle
Le programme de restauration fonctionnelle était réalisé en centre de rééducation fermé, 5 jours par semaine pendant quatre semaines. Il comprenait l’intervention d’un médecin de médecine physique et de réadaptation, d’un psychiatre, de kinésithérapeutes d’ergothérapeutes, d’infirmiers et d’aides soignants. Le programme d’exercices physiques comprenait :
- •
étirements musculaires du tronc et des membres inférieurs ;
- •
renforcement musculaire du tronc et des quatre membres ;
- •
activités aérobies sur bicyclette ergonomique, rameur, tapis de marche et en plein air ;
- •
manutention de charges.
La progression des patients en renforcement musculaire, manutention et activités aérobies se faisait par paliers prédéterminés. Les critères de cette progression étaient le nombre d’exercices par série, la charge, la puissance développée et la durée.
2.2.3
Validation
Les patients ont été évalués sept jours avant le début (j−7), au début (j0) et à la fin (j25) du programme de restauration fonctionnelle. Les critères d’évaluation étaient le questionnaire Roland-Morris , l’indice de Québec et le domaine « activités quotidiennes » du questionnaire de Dallas . L’indice de Québec et le domaine « activités quotidiennes » du questionnaire de Dallas explorent comme le questionnaire Roland-Morris, le retentissement fonctionnel des lombalgies communes. Le questionnaire Roland-Morris est constitué de 24 items permettant de coter la limitation fonctionnelle de 0 à 24 pour les difficultés les plus importantes . L’indice de Québec comprend 20 items pour une limitation maximale cotée à 100 . Le domaine « activités quotidiennes » de l’échelle de Dallas est constituée de 7 items pour une limitation fonctionnelle maximale également cotée à 100 .
La durée de l’étude a été fixée en fonction de l’effectif nécessaire à atteindre. La validation de la version française de l’échelle de Dallas par le Groupe d’étude des lombalgies de la Société française de rhumatologie a été réalisée à partir d’un effectif de 54 patients . Ce nombre fut légèrement majoré pour la validation du questionnaire Roland-Morris afin de tenir compte d’éventuelles sorties d’études ou de données manquantes. Il fut fixé à 60 sur la base de l’inclusion et du traitement de 5 patients par mois pendant 12 mois. L’exploitation des données recueillies a été effectuée en un temps à la fin des périodes d’inclusion et de traitement de l’ensemble de la population étudiée. La validation du questionnaire Roland-Morris a compris l’étude de sa cohérence interne, de sa reproductibilité, de sa validité contre critère et de sa sensibilité au changement.
2.2.4
Cohérence interne
Le questionnaire Roland-Morris appliqué à j0 a été utilisé pour l’étude de la cohérence interne. Le critère de cohérence interne était le coefficient alpha de Cronbach . Une valeur supérieure à 0,60 a été considérée comme satisfaisante.
2.2.5
Reproductibilité
Les questionnaires Roland-Morris appliqués à j−7 et à j0 ont été utilisés pour la reproductibilité. Son estimation a été faite à partir de la méthode de Bland et Altman ainsi que du coefficient de corrélation intraclasse (CCI) et de son intervalle de confiance à 95 % (IC95 %). Un coefficient > 0,60 attestait d’une bonne reproductibilité .
2.2.6
Validité contre critère
Les questionnaires Roland-Morris, de Québec et de Dallas appliqués à j0 ont été utilisés pour l’étude de la validité contre critère. Celle-ci a été estimée à partir du coefficient de Spearman . Un coefficient > 0,5 attestait ici d’une corrélation moyenne, un coefficient > 0,70 d’une bonne corrélation.
2.2.7
Sensibilité au changement
Les questionnaires appliqués à j0 et j25 ont été utilisés pour évaluer la sensibilité au changement. Celle-ci a été estimée dans l’ensemble de la population à partir du test de Wilcoxon avec une valeur de p < 0,05 et à partir de la taille de l’effet observé soit la différence des valeurs obtenues à j0 et j25 divisée par la déviation standard initiale. Des valeurs de 0,2, 0,5 et 0,8 indiquaient une réponse faible, modérée et importante, respectivement . Le pourcentage de variation chez les patients se déclarant améliorés a également été comparé à celui des patients non améliorés par le test de Mann-Whitney.
2.3
Résultats
Cinquante-huit patients consécutifs ont été inclus ( Tableau 1 ). Six d’entre eux l’ont été directement à l’entrée dans le centre de rééducation au début du programme de restauration fonctionnelle et n’ont pas été évalué à j−7. Cinq patients dont deux parmi les précédents n’ont pas rempli le questionnaire Roland-Morris à j25.








