Studies have shown promise in using various approaches of magnetic resonance imaging (MRI) and magnetic resonance spectroscopy to evaluate skeletal muscle involvement in Duchenne muscular dystrophy. However, these studies have mainly been performed using a cross-sectional design, and the correlation of these MRI changes with disease progression and disease severity has not been fully elucidated. Overall, skeletal muscle MRI is a powerful and sensitive technique in the evaluation of muscle disease, and its use as a biomarker for disease progression or therapeutic response in clinical trials deserves further study.
In clinical practice, diseases of the neuromuscular system, particularly those that are of primary muscle origin, are evaluated with history and examination; electrodiagnostic testing (nerve conduction testing and electromyography); genetic testing; and muscle biopsy. Recently, there has been increasing interest in noninvasive imaging modalities, particularly muscle magnetic resonance imaging (MRI), for the diagnosis and assessment of disease progression for various neuromuscular diseases, including Duchenne muscular dystrophy (DMD).
Muscle imaging
Muscle Ultrasound
The use of muscle ultrasound in the assessment of muscle disease began to gain interest in the 1980s. This technique is still used clinically to identify muscle involvement and to aid in the selection of muscles for biopsy. However, the use of muscle ultrasound is limited because this technique is operator-dependent and not all muscles can be adequately assessed.
Muscle MRI and Magnetic Resonance Spectroscopy
MRI is a noninvasive imaging method without ionizing radiation, which has the ability to resolve muscle, fat, connective tissue, and bone. MRI has several advantages over muscle ultrasound, including that MRI has minimal operator-dependence and allows for excellent visualization of all muscles. Magnetic resonance spectroscopy (MRS) is a related noninvasive biochemical sampling technique that has been used in conjunction with MRI to quantify lipid fraction and metabolic products within muscle.
Duchenne muscular dystrophy
DMD is the most common muscular dystrophy affecting children. It is an X-linked recessive disorder with an incidence of 1 in 3300 live male births. The disease is caused by mutations in the gene coding for the dystrophin protein. The dystrophin protein is a member of the dystrophin glycoprotein complex, an essential component of the muscle membrane that provides a link to the extracellular matrix. Mutations in the gene leading to abnormal or absent dystrophin protein lead to absence of localization of dystrophin to the dystrophin-associated glycoprotein complex as seen in muscle biopsy specimens. Disruption of the dystrophin glycoprotein complex and its linkage to the extracellular matrix leads to muscle membrane fragility and renders the myofiber more susceptible to contraction-induced injury. Repeated cycles of myofiber degeneration and necrosis ultimately lead to fatty and connective tissue replacement.
Clinical Course
Boys with DMD typically come to clinical attention before age 5, presenting with delayed motor milestone acquisition, abnormal gait, and difficulty rising from the floor or ascending stairs. Patients experience progressive weakness, beginning in the proximal hip and shoulder girdle muscles, and later progressing to involve more distal muscles. Loss of ambulatory function is usually observed between the age of 10 and 15 years, and by the late teenage years or early twenties most patients die of cardiopulmonary complications.
Diagnosis
The diagnosis of dystrophin-related muscular dystrophy is made by history, physical examination, and markedly elevated serum creatine kinase level, and confirmed by genetic analysis of the dystrophin gene looking for deletions, duplications, or sequence variations.
Current Treatment
Glucocorticoids, a class of corticosteroids, are the only current pharmaceutical agent found to be beneficial in slowing disease progression in boys with DMD and are recommended as standard of care after boys enter the plateau or decline phases of the disease. Steroid therapy has been shown to benefit boys with DMD, with improved muscle strength, prolonged ambulation, stabilized pulmonary and cardiac function, and a reduced incidence of scoliosis. Despite known clinical benefit, the cellular mechanism by which steroid agent stabilizes muscle function has not been clearly elucidated. Although glucocorticoids have been shown to reduce the number of cytotoxic T cells, the immunosuppressant action of this drug is likely not the only mechanism protecting muscle in boys with DMD.
Duchenne muscular dystrophy
DMD is the most common muscular dystrophy affecting children. It is an X-linked recessive disorder with an incidence of 1 in 3300 live male births. The disease is caused by mutations in the gene coding for the dystrophin protein. The dystrophin protein is a member of the dystrophin glycoprotein complex, an essential component of the muscle membrane that provides a link to the extracellular matrix. Mutations in the gene leading to abnormal or absent dystrophin protein lead to absence of localization of dystrophin to the dystrophin-associated glycoprotein complex as seen in muscle biopsy specimens. Disruption of the dystrophin glycoprotein complex and its linkage to the extracellular matrix leads to muscle membrane fragility and renders the myofiber more susceptible to contraction-induced injury. Repeated cycles of myofiber degeneration and necrosis ultimately lead to fatty and connective tissue replacement.
Clinical Course
Boys with DMD typically come to clinical attention before age 5, presenting with delayed motor milestone acquisition, abnormal gait, and difficulty rising from the floor or ascending stairs. Patients experience progressive weakness, beginning in the proximal hip and shoulder girdle muscles, and later progressing to involve more distal muscles. Loss of ambulatory function is usually observed between the age of 10 and 15 years, and by the late teenage years or early twenties most patients die of cardiopulmonary complications.
Diagnosis
The diagnosis of dystrophin-related muscular dystrophy is made by history, physical examination, and markedly elevated serum creatine kinase level, and confirmed by genetic analysis of the dystrophin gene looking for deletions, duplications, or sequence variations.
Current Treatment
Glucocorticoids, a class of corticosteroids, are the only current pharmaceutical agent found to be beneficial in slowing disease progression in boys with DMD and are recommended as standard of care after boys enter the plateau or decline phases of the disease. Steroid therapy has been shown to benefit boys with DMD, with improved muscle strength, prolonged ambulation, stabilized pulmonary and cardiac function, and a reduced incidence of scoliosis. Despite known clinical benefit, the cellular mechanism by which steroid agent stabilizes muscle function has not been clearly elucidated. Although glucocorticoids have been shown to reduce the number of cytotoxic T cells, the immunosuppressant action of this drug is likely not the only mechanism protecting muscle in boys with DMD.
Potential uses of MRI in DMD
MRI Findings in DMD
Numerous studies have shown the ability of MRI to detect alterations in skeletal muscle structure and composition in patients with muscular dystrophy. Initial studies used T1-weighted images and postcontrast imaging to document structural alterations in patients with muscular dystrophy and provide a qualitative assessment of skeletal muscle. Mercuri and colleagues developed a four-point grading system to categorize disease severity, based on visual inspection of fatty tissue infiltration. This strategy was recently used to screen DMD subjects in a clinical trial involving injection with antisense oligonucleotides. However, there is considerable interest in using quantitative imaging to monitor disease progression and efficacy of treatment strategies, which is the focus of the remainder of this article.
T1-Weighted Imaging
Quantitative evaluation with T1 imaging has been used to evaluate MRI changes in DMD. T1 values in DMD subjects are high early in the course of disease and fall with increasing clinical severity, associated with increasing fatty replacement of muscle. More recently, Garrood and colleagues showed that in eight of nine muscles analyzed, there was a statistically significant difference in the median muscle signal intensities on T1-weighted imaging between DMD subjects treated with steroids and control subjects.
T1-weighted imaging and gadolinium enhancement
Gadolinium contrast is used in MRI to better visualize vascular structures. In animal models of muscular dystrophy, gadolinium, which in normal muscle remains extracellular, is taken up into muscle fibers with damaged membranes. Given that membrane damage is present since birth, some hypothesize that this technique may be more appropriate for evaluating DMD patients early in the course of disease, before fatty infiltration is significant. In a recent study, Garrood and colleagues showed a significant increase in gadolinium uptake in the tibialis anterior muscles after stepping exercise in subjects with DMD, but not in the six other muscles studied and no change in the T2 value.
Muscle cross-sectional area
Some studies have used various MRI approaches to measure muscle size in DMD. Muscle cross-sectional area is a quantitative measure, typically calculated from manually tracing individual muscles in axial slices from T1-weighted images ( Fig. 1 ). Recently, Mathur and colleagues used muscle cross-sectional area to compare boys with DMD with age-matched control subjects. They found that muscle cross-sectional area of the posterior calf muscles (soleus, medial, and lateral gastrocnemius) was approximately 60% greater in boys with DMD compared with control subjects, but this finding was not found in the two other muscles tested (tibialis anterior and quadriceps). Furthermore, they showed that there was an age-dependent relationship between the muscle cross-sectional area of the quadriceps muscle, with larger cross-sectional area in boys with DMD compared with control subjects less than the age of 10 years with a reversal of this association in boys aged 11 years and older. Therefore, the rate of progression of disease depends on muscle group, and an advantage of MRI is that numerous muscles can be evaluated at the same time.
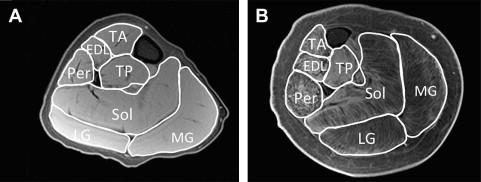
Although the calculation of muscle cross-sectional area is valuable, it does not discriminate between muscle tissue and fatty tissue/edema. These two components can be separated to calculate the muscle contractile and noncontractile tissue within an individual muscle. A recent study used this technique to compare 28 boys with DMD with 10 control subjects. It was shown that boys with DMD had a significantly greater proportion of noncontractile tissue compared with control subjects and that the proportion of noncontractile tissue increased significantly with age. Further, the proportion of noncontractile tissue correlated with a number of functional tests, including time to walk 30 ft; rise from the floor (supine up); and ascend four steps.
T2-Weighted Imaging
Other investigators have used T2-weighted imaging to reflect muscle composition, including damage, inflammation, and lipid composition. T2-weighted imaging has been used to visualize dystrophic lesions in animals, and T2 mapping enables a quantifiable measure that enables more direct comparisons over time and at different sites. Kim and colleagues recently used a T2 mapping strategy to correlate mean T2 values with the nonquantitative MRI grading scale developed by Mercuri and colleagues and with clinical measures of disease severity. They found that the mean T2 of the gluteus maximus muscle showed significant correlation with the nonquantitative MRI score for fatty infiltration. Furthermore, they showed a significant positive correlation between the mean T2 value for the gluteus maximus muscle and several clinical measures, including patient age, clinical function scale, timed Gower score, and time to run 30 ft. In addition, distribution of T2 values in a region-of-interest has been examined on a pixel-by-pixel basis to provide information about muscle heterogeneity. An example of how this may be used to monitor progression of disease is shown in Fig. 2 .










