Cardiac complications are a common feature of many muscular dystrophies. Although many modalities (eg, ultrasound) provide exceptional efficacy for early diagnosis, repeated monitoring, and therapeutic management, MRI has become the gold standard for anatomic and functional characterization. An increasing number of studies, especially in the dystrophinopathies, use strain imaging to evaluate function. This article summarizes these studies and attempts to integrate an understanding of other relevant cardiac features (eg, fibrosis) into interpreting this work. Finally, a general roadmap forward is provided as these tools are increasingly used for treatment assessment and tactical patient management in the future.
Cardiac complications are present in most muscular dystrophies. Although the range of injurious mechanisms is extremely varied, including defined modulating factors (eg, putative genes, signaling, and maintenance), age (or disease progression) at diagnosis and initial treatment, and treatment dosing, the need to characterize disease features with high accuracy is paramount. Because MRI can provide precise chamber volumes from three-dimensional images, and a wide range of other functional metrics, it is considered the measurement gold standard. Paterson and colleagues describe methods other than MRI (eg, CT, positron emission tomography, single photon emission CT). Because dystrophinopathies (eg, Duchenne muscular dystrophy, Becker muscular dystrophy, X-linked dilated cardiomyopathy) represent most of the published work incorporating MRI strain investigation, this literature is summarized to emphasize the current and future role of MRI in disease management.
MRI images focused on anatomy are typically acquired during the diastolic portion of the cardiac cycle to eliminate cardiac motion, with breath-holding generally used to eliminate respiratory artifacts. Multiple averaged acquisitions are another approach to ameliorating respiratory artifacts in patients who cannot perform breath-holds, as is often the case with advanced stages of muscular dystrophy. Specific modification of the sequence parameters produces longitudinal (T1) or transverse relaxation (T2) weighting, which is helpful for characterizing the myocardial tissues. Black blood sequences have been used traditionally for anatomic analysis and generally use electrocardiogram-gated spin echo techniques with successive nonselective and slice-selective inversion pulses, allowing suppression of the blood pool signal with restoration of myocardial signal before image acquisition.
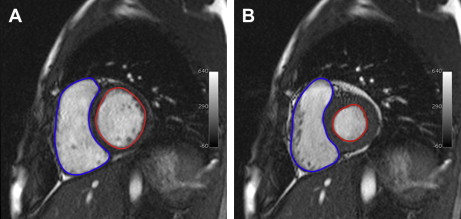
The current workhorse for functional cardiac MRI is the balanced steady-state free precession (SSFP) gradient echo technique, which mainly replaced spoiled gradient echo imaging. SSFP sequences have bright-blood characteristics from T2/T1 effects, and image acquisition is segmented and spread over multiple heartbeats with the resulting cine allowing assessment of the entire cardiac cycle. Myocardial motion is easily depicted with cine sequences typically yielding a temporal resolution in the range of 50 ms (ie, 20 frames per cardiac cycle at a heart rate of 60 beats per minute), which provides a method for subjective assessment of myocardial contractility and wall motion abnormalities. Higher temporal resolutions can be used for valve information. Ventricular contours are traced on individual end systolic and end diastolic images to calculate ventricular volumes using the modified Simpson’s method ( Fig. 1 ). These volumes are used to derive the following usual parameters of ventricular function:
ESV: end systolic volume
EDV: end diastolic volume
SV: stroke volume = EDV – ESV
EF: ejection fraction = (SV/EDV) × 100
Cardiac output; SV × heart rate
Phase contrast techniques use gradient echo techniques to derive quantitative velocity and flow results from the blood pool within the vessel of interest. Various permutations of this technique are helpful in identifying flow disturbances, such as with stenosis, valvular pathology, and intracardiac shunts.
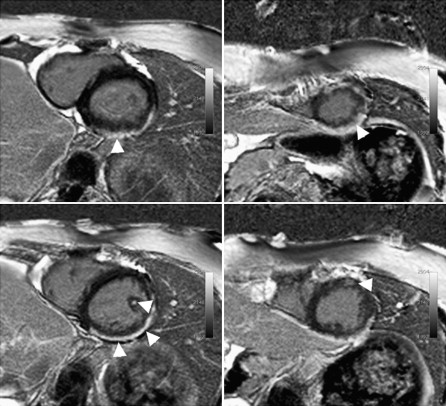
Administration of gadolinium-based contrast agents has efficacy in identifying and characterizing myocardial pathology. Typical gadolinium chelates accumulate within the extracellular space where they shorten T1 and T2 relaxation, with delayed clearance in areas of fibrosis or impaired vascularity. This detail forms the basis of late (delayed) gadolinium enhancement (LGE) techniques now widely used for detecting myocardial infarction and various cardiomyopathies. An example of LGE in a patient with Duchenne muscular dystrophy is shown in Fig. 2 .
Various methods of strain imaging provide an alternate way to quantify myocardial tissue and wall deformation. Most commonly, several slices are sampled in the short-axis plane. The most reported metric derived from these methods is circumferential strain (ε cc ), which is the shortening/wringing motion during systole, whereas other strain parameters, such as radial and longitudinal deformation, are also used. Furthermore, some studies focus on diastolic strain metrics. MRI parameters are principally in units of magnitude, although they can be reformatted to look at coherent changes in time, which is discussed in more detail.
Since its development approximately 20 years ago, strain assessment has been applied to a range of conditions and questions, such as patients with thalassemia ; a healthy cohort to determine relationship between cardiac parameters (eg, volumes) and strain metrics ; and patients after heart transplant. Widespread application has not been realized, however, probably because of the extra time required for examination (making it difficult in ill patients) and the limited availability of free or low-cost postprocessing methods.
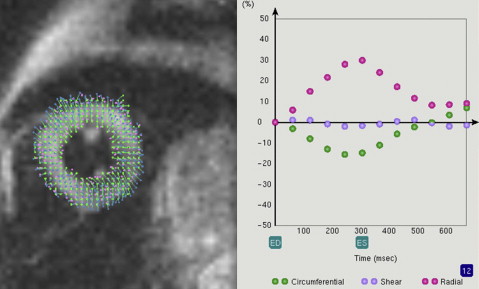
Three main analysis methods are described in the literature: harmonic phase tagging (HARP), which is the most common; fast cine displacement-encoded (DENSE); and cine sequence-based feature tracking (FT ). Processed data then evaluate directional changes over time in cross-hatched “tags,” whereas in DENSE, the information is contained in opposing field gradients. Fig. 3 provides an example of a tagged image. Although HARP/DENSE methods are more similar than different, DENSE is often used for applications focused on greater in-plane resolution, at the expense of signal-to-noise per unit time. FT uses standard cine magnitude images plus segmentation to generate a strain map of the heart walls, with the caveat that this approach excludes a within-tissue component.
Duchenne muscular dystrophy, the most frequent (6/100,000 live births ) and severe of the dystrophinopathies, has been the focus of most articles using cardiac MRI, and strain specifically. Hermans and colleagues provide an excellent survey of broadly focused muscular dystrophy genetics, with attention to cardiac findings. Dystrophin is a large protein that composes part of the normal muscle membrane. In Duchenne muscular dystrophy, the entire reading frame is absent, resulting in complete protein absence, whereas in Becker muscular dystrophy (affecting 2.4/100,000 live births ) the reading frame remains, resulting in abnormally low or variable protein levels. In contrast, X-linked dilated cardiomyopathy does not result in skeletal muscle symptoms, although a rapidly progressive fatal cardiomyopathy occurs in the second decade of life.
The functional consequence of these membrane gaps is that muscle becomes highly vulnerable to injury during normal function. Because of the dramatic injury progression over time in these diseases, research investigation has focused on the early detection of subclinical vulnerabilities in the heart, with the hope that treatment can be initiated to delay or forestall life-threatening consequences.
Strain as an early indicator of heart problems
The Cincinnati group has completed several investigations related to the early use of MRI strain metrics in Duchenne muscular dystrophy. Hor and colleagues focused on a sample of 70 children with Duchenne muscular dystrophy spanning a wide age and range of functional impairment (from normal to impaired EF and LGE) compared with control subjects. Importantly, in the youngest most unaffected (<10 years of age, with no changes in EF or LGE), strain metrics (ε cc ) were abnormal relative to the control group. In older and more symptomatic subjects (eg, EF decreases and positive LGE), strain values were further decreased. These results were replicated in a follow-up study in an expanded sample (N = 191). That study also compared strain metrics from HARP with those generated from postprocessing of the routine cine images (FT). Methods were highly concordant in measuring ε cc in the patient sample (R = 0.854), and when combining the patient group with controls (N = 0.899), supporting the validity of measuring strain from this conventionally acquired sequence. However, a companion letter stated that HARP analyses outperformed feature-based tagging on discriminating young versus old subjects and in terms of accuracy (eg, FT showed a relative underestimation of circumferential strain because the heart wall does not get labeled and tracked). Although these stated concerns are valid, easy implementation of strain with MRI has largely been hampered by the need to acquire and analyze additional specialized sequences. Although the software to perform FT is not freely available, the ability to turn a routine cine sequence into strain metrics hints at the tremendous caches of historical data that could be explored for research purposes.
A follow-up study in an expanded sample (N = 236) included a measure of cross-correlation delay (XCD). XCD is computed from the timing of the ε cc parameters across paired cardiac segments. The analysis then divides the anatomic distribution of XCD into clustered segments (several contiguous segments) or dispersed segments (variable segments around the ring). Results suggested that XCD changes were not common in the sample as a whole, but more frequent (31.2%) in the most affected sample of older boys (N = 16) with EF less than 55% and LGE. The pattern of result was found to be dispersed, a distribution that is noted to have a poor response to resynchronization therapy. Although these methods await further work to evaluate efficacy in other cohorts, the study illustrates the range of metrics that can be achieved with strain analyses.
Complexity of interpreting strain metrics
Strain metrics are not, however, without substantial complexity of interpretation. Ashford and colleagues illustrated this in a small sample of patients with Duchenne muscular dystrophy (N = 13; 11/13 treated with routine long-term corticosteroids), whose circumferential strain (ε cc , HARP), measured at the heart base, mid-ventricle, and apex, was abnormal in a range of cardiac segments (global, septum, anterior, lateral). Older patients had a greater burden of abnormalities, including regional wall changes, ventricular dilation, and decreased left ventricular function. Although anatomic features, such as areas of fibrosis as assessed with LGE, offer one parsimonious explanation for strain differences, diastolic increases compared with normative values were also seen. The authors note that the role of these pre-load effects on heart deformation, perhaps related to corticosteroid use in most patients (11/13), might be an important factor to consider when examining group differences.
Complexity of interpreting strain metrics
Strain metrics are not, however, without substantial complexity of interpretation. Ashford and colleagues illustrated this in a small sample of patients with Duchenne muscular dystrophy (N = 13; 11/13 treated with routine long-term corticosteroids), whose circumferential strain (ε cc , HARP), measured at the heart base, mid-ventricle, and apex, was abnormal in a range of cardiac segments (global, septum, anterior, lateral). Older patients had a greater burden of abnormalities, including regional wall changes, ventricular dilation, and decreased left ventricular function. Although anatomic features, such as areas of fibrosis as assessed with LGE, offer one parsimonious explanation for strain differences, diastolic increases compared with normative values were also seen. The authors note that the role of these pre-load effects on heart deformation, perhaps related to corticosteroid use in most patients (11/13), might be an important factor to consider when examining group differences.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








