Chapter 28 Urinary Organic Acids Profiling for Assessment of Functional Nutrient Deficiencies, Gut Dysbiosis, and Toxicity
 Introduction
Introduction
Urinary organic acid analysis for metabolic profiling was used initially to assess inborn errors of metabolism that can cause severe mental retardation or death within the first year of life. The identification of isovaleric acidemia in 1966 was followed quickly by numerous additional acidurias, because the severe enzyme impairments of genetic diseases were found to result in urinary excretion of highly elevated amounts of organic compounds.1 Profiling of organic acids in urine as a routine laboratory evaluation of chronic diseases is a relatively recent addition to clinical assessment procedures.
Levels of organic acids in urine can be used to detect functional nutritional deficiencies, to identify genetic variants, and to establish the source of toxicants from the environment and gut.2–4 For example, elevated methylmalonic acid (MMA) in urine is a sensitive indicator of functional vitamin B12 deficiency because the vitamin plays a critical role in metabolic clearance of MMA.5,6 Similarly, levels of other organic acids in urine are markers of multiple nutritional deficiencies. Organic acid profiling of urine has been found useful in numerous clinical evaluations, as shown in Table 28-1.
TABLE 28-1 Clinical Applications of Organic Acids in Urine Testing
| CONDITION | COMPOUND(S) | REFERENCE(S) |
|---|---|---|
| Acidosis with metformin | Lactate | 81, 82 |
| Acute appendicitis | 5-Hydroxyindoleacetate | 83 |
| Adult neurologic complaints and breathing problems | Pyroglutamate | 84 |
| Alcoholic delirium tremens | Vanillylmandelate, homovanillate | 85 |
| Anemia | Methylmalonate | 86 |
| Arginine-responsive hyperammonemia | Orotate | 88 |
| Attention deficit hyperactivity disorder | Homovanillate | 87, 89 |
| Bacterial overgrowth syndromes in acutely ill infants and children | p-Hydroxyphenylacetate | 90 |
| Biotin-responsive orificial skin lesions, lethargy, hypotonia, and alopecia | 3-Hydroxyisovalerate | 91 |
| Birth asphyxia | Lactate, pyruvate, 3-hyroxybutyrate, 4-hydroxyphenyllacetate, 2-hydroxybutyrate, 2-oxo-isocaproate, 2-oxo-3-methylvalerate | 92 |
| Calcium urolithiasis | Citrate | 93 |
| Carnitine-responsive familial Reye-like syndrome | Pyruvate, α-ketoglutarate, α-ketoadipate | 94 |
| Cerebral palsy | Fumarate | 95 |
| Chronic fatigue syndrome | Succinate | 96 |
| Chronic lactic acidosis (weakness, shortness of breath) | Alpha-ketoglutarate, α-hydroxybutyrate | 97 |
| Encephalomyelopathy | Alpha-ketoglutarate, succinate, adipate, suberate | 98 |
| Encephalopathy | D-Lactate | 99 |
| Folate deficiency developmental disorders | 5-Hydroxyindoleacetate, homovanillate | 100 |
| Glycine-responsive malnutrition | Pyroglutamate | 101 |
| Gut flora and Klebsiella-specific metabolites | Benzoate, phenylacetate, p-hydrophenylpropionic, dihydroxyphenylacetate | 102, 103 |
| Hemoblastoses and nephroblastoma | p-Hydroxyphenyllactate | 104 |
| Hepatic detoxification | Pyroglutamate | 105 |
| Phenol exposure | o-Cresol | 106 |
| Pregnancy decrease of biotin status | 3-Hydroxyisovalerate | 107 |
| Recent alcohol use | 5-Hydroxyindoleacetate | 108 |
| Riboflavin-responsive inspiratory stridor | Ethylmalonate | 109 |
| Sleep-waking rhythm | Tartrate | 110 |
| Thiamin-responsive maple syrup urine disease | Alpha-ketoisovalerate | 111 |
| Toluene exposure | Hippurate | 112 |
| Vitamin deficiency in the elderly | Homocysteine, 2-methylcitrate | 113 |
| Xylene exposure | 2-Methylhippurate | 114 |
 Urinary Organics Profiling
Urinary Organics Profiling
1. Is mitochondrial energy production adversely affected?
2. Are functional nutrient deficiencies present?
3. Are neurotransmitters displaying abnormal turnover rates?
4. Is antioxidant protection status insufficient?
5. Is toxicant exposure elevated?
6. Are there elevated growth rates of upper intestinal bacteria or fungi?
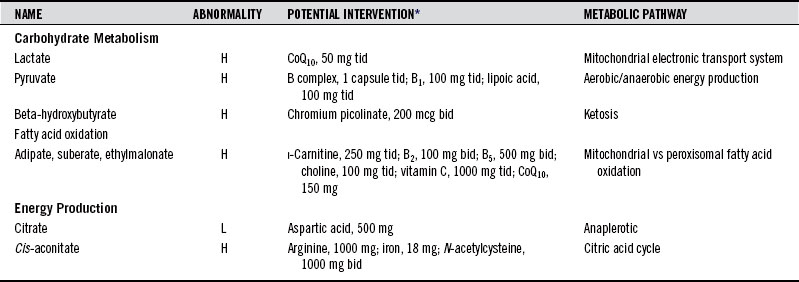
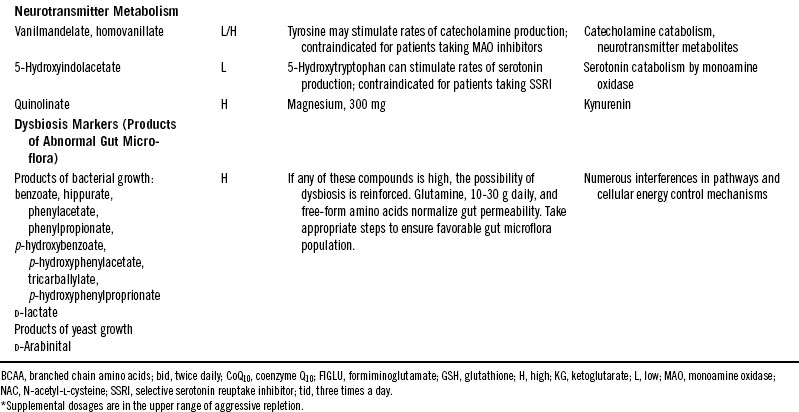
Table 28-2 shows compounds that might appear on a typical quantitative report of organics in urine with some interventions that have been found to help rectify conditions associated with abnormal levels. The supplementary nutrient amounts are given as guides to starting points that might improve clinical outcomes for adults.
 Fatty Acid Oxidation
Fatty Acid Oxidation
Adipate, Suberate, and Ethylmalonate
Adipate and suberate are short-chain dicarboxylic fatty acids produced by an alternate oxidation pathway called ω-oxidation, which occurs in peroxisomes. Their production is normally low because the predominant pathway is β-oxidation in the mitochondria. However, transport of fatty acids into the mitochondria requires carnitine as a carrier. Suboptimal levels of carnitine result in inadequate transfer of fatty acids into the mitochondria and subsequent compensation via ω-oxidation, producing elevated urinary levels of adipate and suberate. Similar carnitine transport impairment leads to elevation of urinary ethylmalonate. Urinary concentrations of all three of these compounds are elevated in overt enzyme failure, but the patterns of elevations in milder carnitine deficiencies vary.
Symptoms include periodic mild weakness, nausea, fatigue, hypoglycemia, “sweaty feet” odor, recurrent infections, and increased free fatty acids. Patients may also exhibit a Reye-like syndrome with dicarboxylic aciduria, which has been associated with various metabolic toxins generated from viral infections that affect mitochondrial function. Mild heterozygotic forms of dicarboxylic aciduria may be commonly seen clinically and may go unrecognized. The enzymes involved also respond to environmental toxin exposure with altered lipid metabolism, which can lead to impaired immune responsiveness.7,8 Supplementation of carnitine and riboflavin is indicated when urinary adipate, suberate, or ethylmalonate is elevated. (See Chapter 74 for a full discussion of this useful nutrient.)
 Carbohydrate Metabolism
Carbohydrate Metabolism
Lactate and Pyruvate
For each molecule of glucose carried through the glycolytic pathway, two molecules of pyruvate are produced. The conversion of pyruvate into acetyl-coenzyme A requires the pyruvate dehydrogenase enzyme complex that is dependent on cofactors derived from thiamin, niacin, riboflavin, pantothenic acid, and lipoic acid. Elevations of pyruvate may reflect failure of the enzyme because of a functional need for increased B vitamins, particularly thiamin and pantothenic acid. Levels of pyruvate in the tissues are further controlled by the biotin-containing protein pyruvate carboxylase, which controls the first step in the formation of glucose from pyruvate. Multiple forms of pyruvate carboxylase deficiency, some of which are biotin responsive, have been reported.9
Any accumulation of pyruvate can lead to concurrent elevations of lactate through the action of lactate dehydrogenase. Lactate accumulates when there is a block in the final oxidative phosphorylation (ox/phos) stage of energy production. Such a block results in inactivation of the citric acid cycle (CAC). Coenzyme Q10 (CoQ10) has been used in cases of lactic acidosis associated with ox/phos impairments.10,11 Increased lactate is a common acidotic condition that can be caused by a variety of metabolic problems. Decreased lactate is seen in people who are physically inactive. Highly trained athletes have such efficient conversion of lactate to glucose that they have lactate levels below the 2.5 percentile.
 Central Energy Pathway Intermediates
Central Energy Pathway Intermediates
Citrate, Isocitrate, Alpha-Ketoglutarate, cis-Aconitate, Succinate, and Fumarate
Citrate, isocitrate, α-ketoglutarate (α-KG), cis-aconitate, succinate, and fumarate are intermediates in the CAC. Most of the enzymes of the CAC require specific vitamin-derived cofactors and minerals for their function. When the enzyme rates slow due to deficiency of their cofactors, abnormal spilling of CAC intermediates into urine can occur. For example, in cytochrome C oxidase deficiency, there is inefficient removal of the primary product of the CAC, reduced nicotinamide adenine dinucleotide, and citrate, malate, fumarate, and α-KG all increase.12 CoQ10 deficiency can also result in elevations of CAC intermediates (see section on Hydroxymethylglutarate). Aggressive dosing with carnitine, CoQ10, and other nutrients has been used to help normalize mitochondrial function in genetic mitochondriopathies.23
Mild inborn errors of energy metabolism that may be compatible with survival, at least into young adulthood, but not with normal development of mental and neurologic functions, have been associated with the excretion of elevated α-KG.24 The clinical heterogeneity of neurometabolic disorders and the importance of organic acid analysis in the diagnosis of static encephalopathy were underscored by the finding of excretion of excess fumaric acid in a 5-year-old girl with a previous diagnosis of cerebral palsy.25
Urinary levels of citrate, a marker of acid-base status, normally decrease with age.26 Metabolic compensation in glycogen storage disease type 1a leads to low urinary citrate concentration and higher incidence of nephrolithiasis. Citrate supplementation may help avoid urinary calculi in patients with low urinary citrate levels.27 Drinking bicarbonate-rich mineral water has also been shown to help raise urinary citrate concentration, and this dietary change has been suggested to prevent the recurrence of calcium oxalate and uric acid stones.28
Intermediates of the cycle can also be derived from amino acids. This may explain the energy-boosting effect that some individuals report when they take free-form amino acid supplements. The effect results from the conversion of specific amino acids directly into CAC intermediates needed for the energy-producing cycle. The fatigue-reducing effect of supplementation of aspartate salts and α-KG acid has been attributed to such a mechanism.9,20 Supplementation of the precursors serves to drive the cycle and generate reducing equivalents used in the electron transport chain in which adenosine triphosphate is produced via oxidative phosphorylation.
Hydroxymethylglutarate
Hydroxymethylglutarate (HMG) is the metabolic precursor of cholesterol and CoQ10. Low levels may reflect inadequate synthesis and possible deficiency of CoQ10. The statin drugs lower cholesterol by blocking the conversion of HMG into mevalonate. They cause elevated urinary HMG and lowered serum CoQ10 levels.21 CoQ10 is used in the mitochondrial oxidative phosphorylation pathway for adenosine triphosphate synthesis and is a potent antioxidant because it lowers the undirected flow of electrons onto molecular oxygen. It has been employed extensively as a cardiovascular protective agent.22
CoQ10 has also been used successfully to improve mitochondrial function in clinical situations of cardiac and skeletal muscle weakness and cramping, and in the extreme, mitochondrial encephalomyopathy.23,24 The coenzyme has been found helpful in the treatment of fatigue, particularly when both lactate and pyruvate are elevated. Such responses reflect the inability of mitochondrial oxidative phosphorylation to proceed efficiently, possibly because of CoQ10 deficiency.
 B-Complex Insufficiency Markers
B-Complex Insufficiency Markers
Alpha-Ketoisovalerate, Alpha-Ketoisocaproate, and Alpha-Keto-Beta-Methylvalerate
Ketoacids derived from the branched chain amino acids isoleucine, leucine, and valine are α-ketoisovalerate, α-ketoisocaproate, and α-keto-β-methylvalerate, respectively. They are formed by the removal of the amine group in branched chain amino acid catabolism and are excreted in large amounts along with their amino acid precursors in the inherited disorder known as maple syrup urine disease. There are several known metabolic disorders in the pathways of catabolism of the ketoacids. The reactions require enzymes similar to pyruvate dehydrogenase that use the B-complex cofactors B1, B2, B3, B5, and lipoic acid. Elevations of the branched chain ketoacids provide a functional assessment of the sufficiency of these vitamins, especially thiamin.25
 Indicators of a Specific Vitamin Deficiency
Indicators of a Specific Vitamin Deficiency
Beta-Hydroxyisovalerate
Beta-hydroxyisovalerate is an ideal marker compound for evaluating biotin status because it is produced in the high-flux pathway for degradation of the most abundant dietary amino acid, isoleucine. The carboxylase enzyme that is necessary for the step that clears β-hydroxyisovalerate requires the active form of biotin; human biotin deficiency has been shown to quickly produce elevation of urinary β-hydroxyisovalerate.26 Beta-hydroxyisovalerate is elevated in inherited disorders of multiple carboxylase insufficiency because biotin is the cofactor for the mitochondrial carboxylase enzymes.27 Symptoms of multiple carboxylase deficiencies include alopecia, rash, Candida dermatitis, unusual odor to the urine, immune deficiencies, and muscle weakness. Biotin deficiencies of various degrees develop in a substantial minority of normal pregnancies and in patients receiving long-term therapy with anticonvulsants.26,28 Other possible indications of biotin deficiency are elevations of lactate and alanine in urine and accumulations of odd-chain fatty acids in plasma or red cell membranes.29 Supplementation of biotin may improve these conditions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree