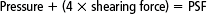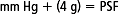CHAPTER 5 Types of skin damage and differential diagnosis
1. Describe the process of at least five factors that contribute to skin damage.
2. Distinguish among the following terms: macule, papule, plaque, nodule, wheal, pustule, vesicle, and bulla.
3. Describe four types of mechanical trauma by the extent of tissue damage associated with each.
4. Discuss at least three interventions for preventing each type of mechanical trauma.
5. Describe three preventive interventions for three common causes of chemical damage.
6. Describe the process of an allergic contact dermatitis.
7. Identify factors that predispose a patient to candidiasis.
8. Describe the types of lesions common to candidiasis, folliculitis, impetigo, and herpes.
9. Discriminate among incontinence-associated dermatitis, candidiasis intertrigo, Stage I pressure ulcer, suspected deep tissue injury (sDTI), perianal herpes, tinea cruris, and inverse psoriasis.
10. Describe the manifestations and care of skin damage due to irradiation.
Skin integrity can be jeopardized or compromised by a multitude of factors: mechanical, moisture, chemical, vascular, infectious, allergy, inflammatory, intrinsic disease, burn, radiation, and miscellaneous assaults. Each type of injury creates a complex set of skin responses, such as erythema, macules, papules, pustules, vesicles/bullae, erosion, and ulcers. Primary lesions of the skin are the first recognizable lesions in the skin. Plate 6 shows the definition and appearance of common primary lesions. Secondary skin lesions evolve from primary lesions due to the natural history of the disease or as a result of scratching/infection. Common secondary lesions are depicted and defined in Plate 7. Because periwound skin can develop skin complications and because many of these conditions provide clues to the etiology of the skin alteration, the health care provider for patients in acute care, home care, outpatient, and long-term care facilities must be familiar with these terms.
Assessment
A systematic skin assessment and an accurate description of any lesions are essential to obtain a reasonable list of differential diagnoses. Additional assessments and diagnostic tests then can be used to derive the most likely diagnosis. Lesions should be described by five morphologic parameters: distribution, shape or arrangement, border and margins, associated changes within the lesion(s), and pigmentation. These parameters, including options for descriptive terminology, are listed in Table 5-1.
TABLE 5-1 Morphologic Characteristics of Skin Lesions
| Characteristic | Description | Examples |
| Distribution | ||
| Localized | Lesion appears in one small area | Impetigo, herpes simplex (e.g., labialis), tinea corporis (“ringworm”) |
| Regional | Lesions involve a specific region of the body | Acne vulgaris (pilosebaceous gland distribution), herpes zoster (nerve dermatomal distribution), psoriasis (flexural surfaces and skin folds) |
| Generalized | Lesions appear widely distributed or in numerous areas simultaneously | Urticaria, disseminated drug eruptions |
| Shape/Arrangement | ||
| Round/discoid | Coin or fine shaped (no central clearing) | Nummular eczema |
| Oval | Ovoid shape | Pityriasis rosea |
| Annular | Round, active margins with central clearing | Tinea corporis, sarcoidosis |
| Zosteriform (dermatomal) | Following a nerve or segment of the body | Herpes zoster |
| Polycyclic | Interlocking or coalesced circles (formed by enlargement of annular lesions) | Psoriasis, urticaria |
| Linear | In a line | Contact dermatitis |
| Iris/target lesion | Pink macules with purple central papules | Erythema multiforme |
| Stellate | Star shaped | Meningococcal septicemia |
| Serpiginous | Snakelike or wavy line track | Cutanea larva migrans |
| Reticulate | Netlike or lacy | Polyarteritis nodosa, lichen planus lesions of erythema infectiosum |
| Morbilliform | Measles-like: maculopapular lesions that become confluent on the face and body | Measles, roseola |
| Border/Margin | ||
| Discrete | Well demarcated or defined, able to draw a line around it with confidence | Psoriasis |
| Indistinct | Poorly defined, have borders that merge into normal skin or outlying ill defined papules | Nummular eczema |
| Active | Margin of lesion shows greater activity than center | Tinea spp. eruptions |
| Irregular | Nonsmooth or notched margin | Malignant melanoma |
| Border raised above center | Center of lesion is depressed compared to the edge | Basal cell carcinoma |
| Advancing | Expanding at margins | Cellulitis |
| Associated Changes within Lesions | ||
| Central clearing | An erythematous border surrounds lighter skin | Tinea eruptions |
| Desquamation | Peeling or sloughing of skin | Rash of toxic shock syndrome |
| Keratotic | Hypertrophic stratum corneum | Calluses, warts |
| Punctation | Central umbilication or dimpling | Basal cell carcinoma |
| Telangiectasias | Dilated blood vessels within lesion blanch completely, may be markers of systematic disease | Basal cell carcinoma, actinic keratosis |
| Pigmentation | ||
| Flesh | Same tone as the surrounding skin | Neurofibroma, some nevi |
| Pink | Light red undertones | Eczema, pityriasis rosea |
| Erythematous | Dark pink to red | Tinea eruptions, psoriasis |
| Salmon | Orange-pink | Psoriasis |
| Tan-brown | Light to dark brown | Most nevi, pityriasis versicolor |
| Black | Black or blue-black | Malignant melanoma |
| Pearly | Shiny white, almost iridescent | Basal cell carcinoma |
| Purple | Dark red-blue-violet | Purpura, Kaposi sarcoma |
| Violaceous | Light violet | Erysipelas |
| Yellow | Waxy | Lipoma |
| White | Absent of color | Lichen planus |
From Seidel HM et al: Mosby’s guide to physical examination, ed 6, St Louis, 2006, Mosby.
Before a treatment plan for any skin lesion or wound can be initiated, the underlying cause of that condition must be determined. Clues to the cause are derived from the patient’s history and physical assessment and specifically by an assessment of the following parameters: location, characteristics, and distribution. These clues can be used to direct subsequent tests that may be necessary to develop a definitive diagnosis. Once the cause of the wound or skin lesion is identified, realistic goals for care can be established and a comprehensive, multidisciplinary treatment plan devised. This chapter introduces a classification system for types of skin damage, briefly describes the pathophysiologic process, and discusses the prevention and treatment of the most common types of skin damage (Box 5-1). The more atypical types of skin lesions, particularly those associated with intrinsic disease, are addressed in Chapter 30.
Mechanical forces
The forces that are applied externally to the skin, such as pressure, shear, friction, and skin stripping (skin tears), create mechanical skin damage. Each may occur in isolation or in combination with other mechanical insults, such as pressure and shear. This chapter presents shear, friction, and skin stripping. Pressure damage is discussed in detail in Chapter 7. Preventive interventions for pressure are discussed in Chapters 8 and 9.
Shear
Shearing force is the sliding movement of skin and subcutaneous tissue while the underlying muscle and bone are stationary (AORN, 2008). This type of skin damage is created by the interaction of tangential forces and friction (resistance) against the surface of the skin. Friction is always present when shearing force is present. The classic example of a shear injury occurs when the patient is in a semi-Fowler position. While the bony structures and muscles slide downward to the foot of the bed, the bed surface generates enough resistance that the skin and subcutaneous tissue over the sacrum remain in the same location (Figure 5-1). Ultimately, the skin is held in place while the skeletal structures pull the body (by gravity) toward the foot of the bed. Consequently, blood vessels in the area are stretched and angulated, the vasculature is disrupted, and small-vessel thrombosis and tissue death may develop (Wright and O’Connor, 2007).
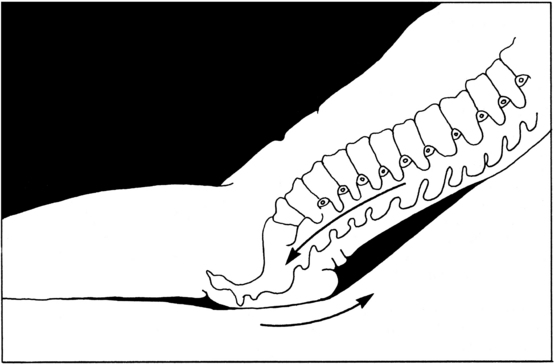
FIGURE 5-1 Shearing force.
(From Loeper JM et al: Therapeutic positioning and skin care, Minneapolis, 1986, Sister Kenny Institute.)
Shear may cause shallow or deep ulcers and extends the tissue damage of pressure ulcers. This extension is manifested in the pressure ulcer by the presence of undermining (dissection or separation of tissue parallel to the skin surface; see Figure 6-4). Shear injury is predominantly localized at the sacrum or coccyx and is commonly a consequence of elevating the head of the bed or improper transfer technique. Prevention requires an awareness of those situations in which the skin is subjected to shearing force. For example, the patient with pulmonary distress requires the head of the bed to be elevated to facilitate adequate ventilation; however, the patient is at great risk for shear injury. Likewise, the patient with a cerebrovascular accident may experience shear injury when being transferred from the bed to the wheelchair. In the operating room, shear may be present with lateral transfers of the patient from the stretcher to the operating table (AORN, 2008).
Most strategies for prevention of shear have derived from expert opinion. Because shear is an important contributing factor to pressure ulcer development, strategies to simultaneously prevent shear and pressure are warranted (see Chapter 8). The primary intervention for reducing shear is the use of lift sheets when repositioning the patient; this eliminates drag on the sacral skin. The head of bed should be maintained at less than 30 degrees; elevations higher than 30 degrees may be needed for meals but should be limited to short periods of time. Also, the knee gatch can be used to interrupt gravity’s pull on the body toward the foot of the bed. Sheepskin may be used; however, its use should not be confused with pressure-redistribution measures. Many support surfaces have a slick fabric covering, which anecdotally and intuitively seems to reduce shear. A standardized method for measuring the ability of a support surface to reduce shear is not currently available.
Fontaineet al (1998) proposed a calculated pressure/shear factor (PSF), which would quantify support surface efficiency for the combined effect of pressure and shear reduction. PSF is calculated by adding the rounded average interface pressure (in millimeters of mercury) to the rounded average gross shearing force (g) multiplied by the impact factor of 4. The equation is as follows:
A pressure sensor and a shear sensor are required to obtain the values to put into the PSF equation. Potentially, PSF could become a tangible support surface measurement. In this way, PSF would provide additional objective information on the potential for the support surface to prevent ulceration. For example, Fontaine et al (1998) studied PSF for three support surfaces classified as group 2 devices according to the Medicare Part B policy (described in Chapter 9). PSF was calculated for a powered, alternating-pressure mattress overlay; a powered, zoned, air-filled mattress replacement device; and a nonpowered fluid overlay. The resulting PSFs were 939, 1,043, and 331, respectively. The implication of this finding is that the nonpowered fluid overlay was more effective in reducing the pressure/shearing force. However, because PSF is a newly defined variable, further research is required to establish the validity of the variable as well as its predictive value and clinical effectiveness.
Friction
The National Pressure Ulcer Advisory Panel (NPUAP) defines friction as the resistance to motion in a parallel direction relative to the common boundary between two surfaces (NPUAP, 2007). Skin injury by friction initially appears as erythema and progresses to an abrasion. As stated previously, shearing force is created by the interaction of tangential forces and friction (resistance) against the surface of the skin. Friction is frequently seen on elbows or heels because the patient easily abrades these surfaces against sheets when repositioning. Injury is characteristically very shallow and limited to the epidermis. Friction primarily affects superficial layers, and thus does not result in tissue necrosis, while shearing forces mainly affect deeper tissue layers (WOCN, 2010).
Skin stripping and lacerations (epidermal and dermal)
Skin stripping is the inadvertent removal of the epidermis, with or without the dermis, by mechanical means. Trauma such as tape removal, electrode removal, or bumping into furniture and assisting with repositioning or mobility can precipitate skin tears (Meuleneire, 2002; Payne and Martin, 1990). For most patients skin tears do not extend hospital stay, but they are painful and distressing in appearance and can be interpreted as being the result of poor care (Ratliff and Fletcher, 2007). In the neonate or premature infant, however, skin tears are a significant portal of entry that can lead to septicemia (Furdon, 2003). Unique considerations concerning skin tears in the neonate or premature infant are addressed in Chapter 36. Data suggest that skin tears heal in 7 to 21 days, depending on the extent of tissue damage (Milne and Corbett, 2005; Meuleneire, 2002).
Although never tested for validity or reliability, the Payne-Martin classification system for skin tears is an instrument commonly used to classify skin tears (Table 5-2). Category I skin tears are distinguished by a resulting skin flap or avulsed skin that can cover the exposed wound (Plates 8–9). Category II wounds (Plates 10–11) are distinguished by the degree of damage to the epidermal avulsed skin. Category III lesions (Plate 12) have no epidermal flap. Typically, skin stripping lesions are irregularly shaped and shallow, involving only the epidermis. Only category IA lesions are full thickness (involve the dermis). The frequency of various locations of skin-stripping injuries is as follows: upper extremities (73%–80%), legs and feet (20%), head (3%–4%), and torso (3%) (Malone et al, 1991; McGough-Csarny and Kopac, 1998; Payne and Martin, 1990). In 2006, the Pennsylvania Patient Safety Reporting System (PA-PSRS) reported similar results and further specified the involved sites on the upper extremities (Pennsylvania Patient Safety Authority, 2006). PA-PSRS reports the most frequent site of injury is the forearm, followed by the arm, hand, and lower extremities (Pennsylvania Patient Safety Authority, 2006).
TABLE 5-2 Skin Tears: Definition and Payne-Martin Classification System
| Category | Subcategory | Description |
|---|---|---|
| I: Skin tear can fully approximate wound | A: Linear skin tear | Full-thickness wound that occurs in wrinkle or furrow of skin. Both epidermis and dermis are pulled apart as if an incision has been made, exposing tissue below. |
| B: Flap-type skin tear | Partial-thickness wound in which the epidermal flap can be completely approximated or approximated so that no more than 1 mm of dermis is exposed. | |
| II: Skin tear with partial-thickness loss | A: Scant tissue loss | Partial-thickness wound in which 25% or less of the epidermal flap is lost and at least 75% or more of the dermis is covered by the flap. |
| B: Moderate to large tissue loss | Partial-thickness wound in which more than 25% of the epidermal flap is lost and more than 25% of the dermis is exposed. | |
| III: Skin tear with complete tissue loss | Partial-thickness wound in which an epidermal flap is absent. |
From Payne RL, Martin ML: Defining and classifying skin tears: need for a common language, Ostomy Wound Manage 39(5):16, 1993.
Risk factors for a skin tear include advanced age, sensory loss, dehydration and malnutrition, history of previous skin tears, cognitive impairment, dependency, poor locomotion, and presence of ecchymosis (McGough-Csarny and Kopac, 1998; Lablanc et al, 2008). However, it is important to remember that even independent, ambulatory patients are at risk for this injury; they experience the second highest number of skin tears (Baranoski, 2001). In this population, patients often have edema, purpura, or ecchymosis, and the skin tears occur primarily on the lower extremities.
Daily care activities, such as bathing, dressing, transfers, and toileting, all require frequent handling of the patient with vulnerable skin. Therefore their potential for inducing a skin tear increases. Use of equipment (e.g., mechanical lifts, wheelchairs, and geriatric chairs) also increases the patient’s exposure to potential trauma, which may precipitate a skin tear (McGough-Csarny and Kopac, 1998).
A standardized care plan for patients at high risk for skin tears is given in Box 5-2 (Ayello, 2003; Lablanc et al, 2008; White et al, 1994). Recognizing “at-risk” individuals and implementing key preventive strategies has been shown to decrease the incidence of skin tears (Bank and Nix, 2006; Brillhart, 2005). Extreme care and a gentle touch are critical when touching the patient or performing patient care, because most skin tears occur during the course of providing routine patient care activities (e.g., bathing, dressing, transferring) (Malone et al, 1991; McGough-Csarny and Kopac, 1998; White et al, 1994). Because harsh soaps and frequent bathing can reduce the skin’s natural lubrication and lead to dry skin, gentle skin cleansers and frequent moisturizing are important components of skin tear prevention (Bank and Nix, 2006; Birch and Coggins, 2003; Brillhart, 2005; Hunter et al, 2002; Ratliff and Fletcher, 2007). It may be necessary to decrease bathing schedules and use humidifiers to increase environmental moisture (Lablanc and Baranoski, 2009; Lablanc et al, 2008). Rooms should be adequately lit to reduce the risk of bumping into furniture and equipment (Ratliff and Fletcher, 2007; Nazarko, 2005) Beyond these measures, the current focus of prevention is on the application of products and garments that serve as barriers between the skin and potential trauma; such products include commercially available skin sleeves, roll gauze, pants, and long-sleeve shirts (Bank and Nix, 2006).
BOX 5-2 Standardized Care Plan for Patients at High Risk for Skin Tears
1. Provide a safe environment.
2. Maintain nutrition and hydration.
3. Protect from self-inflicted injury or injury incurred during routine cares.
When frequent tape removal is needed for dressing changes, application of an adhesive barrier (e.g., solid-wafer skin barrier or thin hydrocolloid) can be used on periwound skin to anchor tape and prevent skin stripping. The protocol should clearly indicate that the barrier is not changed routinely. The barrier should be left undisturbed and allowed to fall off. Loose edges should be clipped rather than removing the old barrier and applying a new one. Used in this way, the skin barrier or hydrocolloid dressing can remain in place for several days while caregivers easily apply and reapply the tape without traumatizing the epidermis. Box 5-3 summarizes interventions for preventing skin tears in conjunction with the use of adhesives and tapes.
BOX 5-3 Prevention of Skin Stripping from Tapes and Adhesives*
1. Secure dressings with roll gauze, tubular stockinette, or self-adhering tape (avoids unnecessary tape on skin).
2. Apply tape without tension (prevents blistering of skin under tape).
3. Use porous tapes (allows moisture to evaporate).
4. Use skin sealants, thin hydrocolloids, or solid-wafer skin barriers under adhesives (provides protective layer over skin for adhering tapes).
5. Secure dressings with Montgomery straps (prevents repeated tape applications).
6. Remove tape slowly, peel away from anchored skin or pull one corner of tape at an angle parallel with skin. Solvents can be used to break bond with skin, although solvents have drying effect on skin. Plain tap water can often serve this purpose effectively (decreases trauma to epidermis and dermal–epidermal junction).
Neonates (See also Chapter 36)
• Solvents and adhesive removers should not be used with the neonate due to reports of skin toxicity.
• Skin sealants should not be used with neonates less than 30 days old. Alcohol free skin sealants may be used for the neonate greater than 30 days old.
Ideally, category I skin tears can be treated by repositioning a residual flap over the wound surface to maintain viability and facilitate reattachment (Lablanc et al, 2008; Ratliff and Fletcher, 2007). The skin flap can be approximated and secured with Steri-strips. The strips should be used judiciously because traction on the skin (even from Steri-strips) can cause further damage (Ratliff and Fletcher, 2007). Approximated edges also may be secured with 2-ocetyl-cyanoacrylate (also referred to as topical skin bandage or skin glue) (Baranoski, 2003; Fleck, 2007; Lablanc et al, 2008; Roberts, 2007).
For treatment of category II and III skin tears, nonadherent dressings or topical liquid films are recommended to minimize tissue trauma and pain when the dressings are removed (Meuleneire, 2002; Milne and Corbett, 2005; Roberts, 2007). Thomas et al (1999) reported that dressings with a high moisture vapor transmission rate, such as those with a low-adhesive foam dressing, result in complete healing of 94% (16/17) category II and III skin tears within 21 days. This is in comparison with complete healing of only 65% (11/17) of skin tears when film dressings are used. The researchers suggest that film dressings keep the skin tear excessively moist and exacerbate the separation of epidermis from dermal papillae. These results underscore the importance of selecting dressings based on the “needs” of the wound or the wound characteristics (see Chapter 18).
Skin tears cannot be prevented by one individual. Because the typical individual at risk for skin tears is dependent on caregivers for many aspects of care, preventive interventions must be embraced by all staff members. Therefore staff education is a critical component of a skin tear prevention and treatment program. By using a web-based educational program, McTigue et al (2009) found that acute care registered nurses from two affiliated hospitals (N = 416) had significantly improved their ability to identify, assess, classify, and treat skin tears (p <.001) after they had completed the program.
Moisture and chemical factors
The nomenclature used to describe and classify skin damage caused by these conditions is extensive and confusing. For example, dermatitis caused by chemical irritants has been referred to as irritant contact dermatitis. The term diaper dermatitis, familiar to most health care providers, specifically refers to inflammation of the skin in the diaper area (perineal or perigenital areas) of the infant (Zulkowski, 2008). However, when the same condition develops in the perineal area in the adult, it is often called perineal dermatitis. The dilemma with both terms is that they do not adequately address the irritant, and the terms exclude locations outside of the diaper or perineum, such as the inner thighs or buttocks.
Recognizing the multiplicity and limitations of terms, a panel of six clinical experts recommended replacing these terms with incontinence-associated dermatitis (IAD), defined as a reactive response of the skin to chronic exposure to urine and fecal material, which could be observed as inflammation and erythema with or without erosion or denudation (Gray et al, 2007a). This term describes the response of the skin (dermatitis, which is inflammation and erythema with or without erosion or denudation), specifically identifies the source of the irritant (urine or fecal incontinence), and acknowledges that a larger area of the skin is commonly affected (Plate 13). Coexisting infections may develop with IAD, most often candidiasis. Additional infections that can arise include herpes zoster or herpes simplex virus (HSV), candidal intertrigo, tinea cruris (a dermatophyte infection of the squamous cells and trapped in a skin fold), and inverse psoriasis (Visscher, 2009).
Skin damage due to moisture on the skin is not limited to the etiology of incontinence. During a consensus meeting of four clinicians addressing the problem with adequate terminology for this condition, the term moisture-associated skin damage (MASD) was coined as a generic term to refer to skin damage in any location on the body that occurs due to exposure to moisture “and associated irritants” (Gray and Weir, 2007; Gray et al, 2007b). Conditions identified by the group to be associated with MASD included periwound maceration, IAD and candidiasis. However, many more moisture-related skin problems probably can be added to this list, including Stage I and II pressure ulcers, intertrigo, and tinea cruris.
From a practical standpoint, it is helpful to discuss skin damage or dermatitis from the perspective of the agent triggering the response in the skin regardless of the anatomic body location. In this manner, the etiology of the skin damage is identified, and appropriate interventions for prevention and treatment are derived. For example, fecal content has a stronger association with skin damage than does urine, which may impact the choice of preventive interventions (Bliss et al, 2006; Junkin and Lerner-Selekof, 2007). In this textbook, we refer to moisture as a factor that weakens intact skin but does not cause breaks in skin integrity. Moisture (e.g., urine, perspiration) is not caustic; the skin generally does not become eroded when exposed to moisture. Conversely, chemicals (e.g., gastrointestinal secretions, stool, harsh cleansing solutions and solvents) are caustic to the skin due to their acidic pH, or high volume of enzymes, and will erode the top layers of the epidermis.
Moisture
Clinical manifestations of skin macerated by the presence of excess or prolonged moisture include pale skin color and a wrinkling, swollen appearance of the exposed skin surface (Plate 14). As the length of exposure continues, the skin may become erythematous, and eventually fissures may develop. For example, linear shallow fissures may develop in the cleft between the buttocks and between the toes.
Maceration compromises protective skin mechanisms such as pH and normal flora. Ammonia in urine also raises the skin’s pH, and changes in skin flora occur. As irritants penetrate the epidermis, they interact with keratinocytes and fibroblasts stimulating the release of cytokines, which act on the vasculature of the dermis to trigger inflammation. Consequently, the lipid bilayer structure of the stratum corneum is damaged, which may allow microorganisms to enter the epidermis (Visscher, 2009). Prolonged moisture also reduces the ability of the skin to resist additional stresses, such as shear, friction, and pressure, because of a higher frictional coefficient (Visscher, 2009) leading to secondary skin breakdown.
Chemical factors
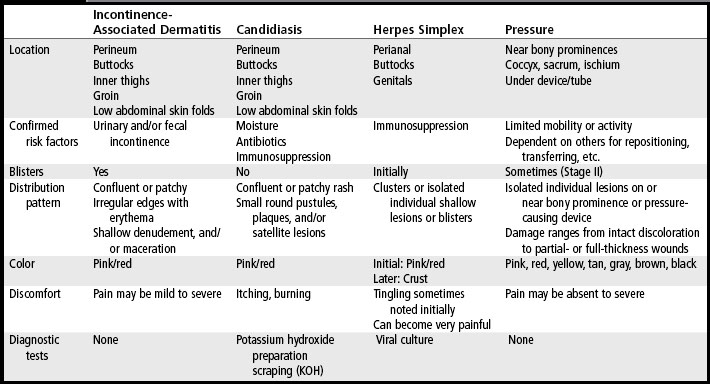
Chemical dermatitis can be distinguished from maceration by examining the exposure sites. Initially, irritants extract water-binding chemicals and lipids from the stratum corneum, and the skin decompensates so that it becomes dry and erythematous or develops an erythematous macular rash (Habif, 2004) (Plate 13). With continued exposure to chemical irritants, the protective layer of the stratum corneum becomes damaged, resulting in a loss of epidermis in the area of exposure which is described as superficial erosion or moist denudement. In contrast, skin with moisture-related damage most often remains intact. Chemically induced dermatitis is uncomfortable for the patient because the chemicals often stimulate neurocutaneous pain receptors. In differentiating chemical dermatitis from the possible skin conditions in the perineal, perianal, or buttocks region, it is also important to recognize that chemical dermatitis will only appear in the area that is directly in contact with the irritant. To facilitate further distinctions, Table 5-3 lists the typical assessment features of a variety of perineal–perianal skin conditions, such as IAD, candidiasis, HSV, herpes zoster, and pressure ulcers.
Acute fecal incontinence is a common instigator of chemical dermatitis. The etiology of acute fecal incontinence can be the result of a number of factors: nutritional (hyperosmolar enteric solutions, rapid rate of administration of enteric solutions, hypoalbuminemia), medications (antibiotics, cathartics), gastrointestinal function (short bowel syndrome, fat malabsorption, antibiotic-associated diarrhea, incomplete bowel obstruction, fecal impaction), and gastrointestinal disease (inflammatory bowel disease, infection, radiation enteritis). The patient’s medical history is helpful in identifying the most likely etiology; additional laboratory tests or radiologic examinations may be indicated for confirmation. A digital rectal examination should be conducted to rule out a fecal impaction causing an incomplete bowel obstruction. When a fecal impaction is present, the stool will be dry and hard, and the patient will not be able to pass the stool unassisted; diarrheal stool will be passed around the impaction (Abeloff et al, 2008). When an impaction is present, a sodium phosphate enema (i.e., fleet enema) and oral cathartics may be indicated.
Antibiotic-associated diarrhea is an increasingly frequent cause of fecal incontinence. Broad-spectrum antibiotics, such as fluoroquinolones, amoxicillin, clindamycin, and cephalosporins, are the most common culprits (Imhoff and Karpa, 2009). Antibiotics alter the gastrointestinal microflora, thus increasing the concentration of pathogenic organisms within the bowel. The predominant pathogens in antibiotic-associated diarrhea are Clostridium difficile, Staphylococcus aureus, and Clostridium perfringens. C. difficile is the most common of these pathogens and is more severe than Escherichia coli and Salmonella (Rohde et al, 2009). C. difficile, an opportunistic spore-forming, gram-positive bacillus, is transmitted via the fecal–oral route, and the spores remain on surfaces for extended periods of time. Only bleach is effective at killing C. difficile on surfaces. C. difficile–associated diarrhea (CDAD) has become the most common nosocomial diarrheal pathogen in hospitalized patients; from 20% to as much as 50% of antibiotic-associated diarrhea is attributed to C. difficile (Asha et al, 2006).
Antibiotic-associated diarrhea/CDAD often begins 4 to 9 days after the antibiotic is stopped but can develop up to 8 weeks later (Rohde et al, 2009). Symptoms of CDAD include watery diarrhea, abdominal cramping and tenderness, abdominal distention, fever, leukocytosis, nausea, and dehydration. Significant inflammation of the colonic mucosa develops primarily as a consequence of the adverse actions of two toxins: toxin A and toxin B. Both secretory diarrhea and osmotic diarrhea occur with CDAD (Eddins and Gray, 2008). As the mucosal surface is damaged, liquid accumulates in the bowel lumen, resulting in secretory diarrhea. Toxins A and B attract proinflammatory cytokines, which further damage the bowel wall and impair its ability to absorb water, electrolytes, and nutrients, thus precipitating osmotic diarrhea (Eddins and Gray, 2008).
C. difficile infection is confirmed by clinical presentation of symptoms (usually diarrhea), exclusion of other causes of diarrhea, and a culture or positive toxin assay (McFarland, 2009). Toxin assay is the preferred method of detecting C. difficile. Most commonly used is the rapid enzyme immunoassay, which detects toxin A or toxin A plus toxin B. Results are available within 2 to 3 hours, although many false-negative results are reported (Bartlett, 2008; McFarland, 2009). The more sensitive tissue culture assay also can be done to detect cytotoxin or toxin B, but results take longer. A direct stool polymerase chain reaction assay provides quick results and reports fewer false-negative results than the enzyme immunoassay (McFarland, 2009). A stool culture for C. difficile is possible but results are not available for 3 days. Before C. difficile toxin assays were available, colonoscopic examinations were common; a colonic biopsy positive for pseudomembranes in the presence of diarrhea is diagnostic of CDAD (Bartlett, 2008).
Standard treatment of CDAD is (1) prompt discontinuation of the antibiotic, (2) stool toxin assay, (3) oral metronidazole (500mg three times per day for 10 days), (4) correction of fluid and electrolyte imbalance, and (5) discontinuation of antiperistalsis medications. Metronidazole should be started even before the stool culture results are available; the systemic symptoms present create a high level of suspicion of CDAD. If abdominal pain persists or the patient does not respond to metronidazole therapy, oral vancomycin (125 mg four times per day for 10 days) is warranted. After initial antibiotic therapy, almost one fourth of patients with CDAD relapse within 2 months because spores can prevent peristalsis and delay exposure to antibiotics by “hiding” in the mucosal folds (Rohde et al, 2009). A more virulent strain has been discovered and is associated with increased disease severity and death (Redelings et al, 2007).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree