Tuberculosis
Jeffrey R. Starke
Tuberculosis remains the most important chronic infectious disease in the world in terms of morbidity, mortality, and cost. An estimated 2 billion people worldwide are infected with the tubercle bacillus, and 1 to 3 million deaths from tuberculosis occur annually. Children in developing countries account for 1.3 million cases and 450,000 deaths annually from tuberculosis. In the United States, an estimated 10 to 20 million people have latent tuberculosis infection, 16,000 people develop tuberculosis each year, and 2,000 die with complications of the disease.
After Mycobacterium tuberculosis enters the lung and begins to multiply, the person has tuberculosis infection. The hallmark of tuberculosis infection is a positive tuberculin skin test result, but chest radiography is normal or reveals only a healed granuloma, and the child is free of signs and symptoms. Tuberculosis disease occurs when clinical manifestations of pulmonary or extrapulmonary tuberculosis become apparent by chest radiography or clinical findings. The word tuberculosis usually refers to disease. Most untreated infected adults never develop disease. The time between the onset of tuberculosis infection and the beginning of disease may be several weeks or many years. In adults, usually a clear distinction between infection and disease exists. However, among children in whom disease usually develops as an immediate complication of the primary infection, the two stages are less distinct. An infected child with radiographic or clinical manifestations consistent with tuberculosis is considered to have disease, even if no symptoms are present.
ETIOLOGY
The genus Mycobacterium is classified in the order Actinomy-cetales and the family Mycobacteriaceae. The major agents of
human tuberculosis are M. tuberculosis and M. bovis. Infections caused by M. bovis are rare in most areas the United States because of the slaughter of infected cattle and the almost universal pasteurization of milk. However, in San Diego, California, one-third of culture-proven cases of tuberculosis in children are caused by M. bovis, the initial infection likely occurring in another country before immigration to the United States.
human tuberculosis are M. tuberculosis and M. bovis. Infections caused by M. bovis are rare in most areas the United States because of the slaughter of infected cattle and the almost universal pasteurization of milk. However, in San Diego, California, one-third of culture-proven cases of tuberculosis in children are caused by M. bovis, the initial infection likely occurring in another country before immigration to the United States.
Tubercle bacilli are nonmotile, non-spore-forming, pleomorphic, weakly gram-positive, curved rods approximately 2 to 4 μm long. They may appear beaded or clumped. They are obligate aerobes and grow in simple synthetic media with glycerol or other compounds as the carbon source and ammonium salts as the nitrogen source. The bacilli grow best at 37°C to 41°C, have a characteristic colony morphology, and lack pigmentation. They often grow as intertwining, serpentine cords.
A hallmark of mycobacteria is acid fastness (i.e., the capacity to form stable mycolate complexes with arylmethane dyes such as carbol-fuchsin, crystal violet, auramine, and rhodamine). The dyes are not removed readily by rinsing with ethanol plus hydrochloric acid. The bacilli appear red when stained with fuchsin (i.e., Ziehl-Neelsen or Kinyoun stains) and purple when stained with crystal violet; they exhibit yellow-green fluorescence under ultraviolet light when stained with auramine and rhodamine (Truant stain).
The cell wall of mycobacteria contains 20% to 60% lipids bound to proteins and carbohydrates. This lipid-rich cell wall accounts for hydrophobic properties, acid fastness, and resistance to the bactericidal actions of antibody and complement. True waxes, mycolic acid, and glycolipids are unique to the cell wall of mycobacteria.
Identification of mycobacteria is based on their growth characteristics, staining properties, and biochemical or metabolic characteristics. Speciation depends on the following: the temperature of optimal growth; catalase production, which is present in virulent, isoniazid-susceptible M. tuberculosis but absent in some isoniazid-resistant strains; the secretion of niacin, which is characteristic of M. tuberculosis; sensitivity to sodium chloride; the reduction of tellurite; and the capacity to produce carotenoid pigments on exposure to light (i.e., photochromogen), equally in light and dark (i.e., scotochromogen), or not at all (i.e., nonphotochromogen). In most modern mycobacteriology laboratories, the identification of M. tuberculosis is established by a DNA probe of colonies on a plate, organisms in broth, or high pressure liquid chromatography analysis of the mycolic acids, which are unique to each strain of mycobacteria.
EPIDEMIOLOGY
The incidence of tuberculosis in the United States declined 5% to 6% each year for several decades until 1985, when it leveled. From 1986 to 1992, the reported number of cases increased to 26,000 per year. From 1992 to 2003, case numbers again declined to approximately 15,000 in 2004. From 1985 through 2003, more than 90,000 additional cases of tuberculosis were reported in the United States than would have been expected if the previous decline had continued. Four major factors often are cited to explain the increase from 1986 to 1992. One cause was the human immunodeficiency virus (HIV) epidemic because coinfection with HIV infection is the greatest risk factor known for the development of tuberculosis disease in an adult infected with the tubercle bacillus. An increasingly important factor was the increase in the number of immigrants to the United States from countries with a high prevalence of tuberculosis. In 2003, more than 50% of U.S. tuberculosis cases occurred among foreign-born patients, compared with 22% in 1985. A third factor, verified by new methods of DNA typing of organisms, was the discovery of transmission of M. tuberculosis in a variety of settings, including hospitals, nursing homes, schools, churches, and bars. Finally, the general decline in public health services and access to medical care in parts of the United States hindered identification of high-risk individuals, rapid diagnosis, treatment, and completion of contact investigations.
In the early twentieth century, the risk of being exposed to an adult with infectious tuberculosis was higher and more uniform across the entire population than it is currently. Tuberculosis has retreated into fairly well-defined groups of high-risk persons (Table 179.1). Cities with populations of more than 250,000 account for 18% of the nation’s population but more than 42% of its tuberculosis cases. Case rates also are high in the Appalachian Mountain region and along the southern border of the United States.
TABLE 179.1. HIGH-RISK GROUPS FOR TUBERCULOSIS IN THE UNITED STATES | |
|---|---|
|
Although tuberculosis case rates in the United States generally have increased with patient age, a trend toward an increased case rate has occurred in young adults, especially among urban minority populations. Case rates are always lowest among children 5 to 14 years of age; most childhood tuberculosis occurs among children younger than 5 years.
Certain environments in our society contain sizable numbers of adults at high risk for developing tuberculosis, which promotes its transmission. Tuberculosis rates in jails, prisons, nursing homes, homeless shelters, and migrant camps often are 10 to 50 times higher than in the general community. Many of these environments house persons at increased risk for acquiring HIV infection. HIV-seronegative adults with untreated tuberculosis infection have a 5% to 10% lifetime risk of developing tuberculosis disease. HIV-seropositive adults also infected with the bacillus develop tuberculosis at a rate of 5% to 10% per year. Although fewer cases of children with coexisting HIV infection and tuberculosis have been reported, the increased rate of childhood tuberculosis from 1986 to 1992 could be linked partially to the spread of tuberculosis infection from HIV-infected adults with tuberculosis.
Children with tuberculosis represent 5% to 6% of the total annual number of cases. Since 1976, the decline in incidence of childhood tuberculosis had been slower than that for older populations. Between 1987 and 1991, the number of pediatric tuberculosis cases in the United States increased by 39%, to 1,656 per year. This increase undoubtedly was linked to the increased case rates among young adults. From 1992 to 2003, the number of tuberculosis cases in U.S. children again declined slightly. The pediatric tuberculosis case rate reflects the
effect of the disease on childhood health and serves as a public health marker of ongoing tuberculosis transmission within a community. As long as the disease persists in adults, susceptible children will become infected.
effect of the disease on childhood health and serves as a public health marker of ongoing tuberculosis transmission within a community. As long as the disease persists in adults, susceptible children will become infected.
Although the number of cases of childhood tuberculosis has increased, the epidemiology in the United States has remained fairly constant. Approximately 60% of childhood cases occur in children younger than 5 years old. The disease affects both genders equally. Although most children acquire infection with M. tuberculosis in their home or neighborhood from relatives or adult family friends, epidemics of childhood tuberculosis, almost always caused by contact with an infectious adult, continue to occur within schools, churches, day-care centers, and nursery schools.
M. tuberculosis is transmitted from person to person, usually by droplets of mucus that become airborne when an infected person coughs, sneezes, laughs, or sings. The duration of exposure required to transmit M. tuberculosis depends on the infectiousness of the source case. Adults with cavitary disease harbor the greatest number of tubercle bacilli for the longest time. The best predictor of contagiousness is a positive acid-fast stain of sputum from an adolescent or adult with pulmonary disease. Most adults are no longer infectious after several days to 2 weeks of therapy, but this period may increase for patients with advanced cavitary disease who continue to cough. Children with pulmonary tuberculosis rarely infect other children. Tubercle bacilli are sparse in the endobronchial secretions of children, who usually do not cough with sufficient force to transmit infection. However, those rare children who develop adult-type tuberculosis, with upper lobe infiltrate or cavity, severe cough, and sputum production, may be infectious to others.
PATHOGENESIS
The primary (Ghon) complex of tuberculosis consists of disease at the portal of entry and the regional lymph nodes that drain the area of the primary focus. The infection may occur anywhere in the body, but the primary site is the lung in more than 95% of cases. Tubercle bacilli on particles larger than 10 μm usually are caught in the mucociliary mechanisms of the bronchi and are expelled. Smaller particles are inhaled beyond the clearance mechanisms. In the alveoli or alveolar ducts, bacilli multiply, and an inflammatory exudate is present. Some of the bacilli are carried by macrophages through the lymphatic channels to the regional lymph nodes. While the primary complex is developing, tubercle bacilli spread by the bloodstream and lymphatics to many parts of the body. This dissemination can involve large numbers of bacilli, leading to miliary tuberculosis or, more commonly, small numbers of bacilli that leave tuberculous foci scattered in various tissues. These foci may or may not develop into significant extrapulmonary tuberculosis later in life. After 4 to 8 weeks, cell-mediated immunity develops. At this time, the primary complex usually heals to the extent that it is not visible on chest radiography, and further dissemination is arrested. These events usually produce no signs or symptoms.
The parenchymal portion of the primary complex often heals completely after undergoing caseating necrosis and encapsulation. Further healing occurs by fibrosis or calcification. The nodal component has a decreased tendency to heal completely. Even after calcification occurs, viable tubercle bacilli may persist for many years in the node or in distant sites. Although children usually develop tuberculosis disease during the primary infection, most cases in adults are caused by endogenous regrowth of latent bacilli resident in the body from the time of the primary infection (i.e., adult or postprimary tuberculosis). The most common form of postprimary tuberculosis affects the apical region of the lung. The apex of the lung has the highest oxygen tension, and the many organisms deposited there during hematogenous dissemination are those most likely to reactivate.
Without specific therapy, tuberculosis disease develops in 5% to 10% of immunologically normal adults with tuberculosis infection at some time during their lives. The risk for children is greater; as many as 40% of children younger than 1 year of age with untreated tuberculosis infection develop radiographic evidence of disease, compared with 24% of children between the ages of 1 and 5 years and 15% of those between the ages of 11 and 15 years. Although infants and young children are more likely to develop immediate complications of the initial infection, children who are older when infected are more likely to develop postprimary disease as adults.
A predictable timetable of tuberculosis infection and its possible complications exists. Massive lymphohematogenous spread leading to miliary or acute meningeal tuberculosis occurs approximately 2 to 6 months after the initial infection develops. Endobronchial tuberculosis, with segmental pulmonary changes, occurs within 9 months. Clinically important lesions of bones or joints do not appear until at least 1 year after infection develops, and renal lesions develop 5 to 25 years after initial infection develops. In general, complications in children occur within the first 5 years (especially the first year) after they develop the initial infection. Complications later in life are caused by reactivation of previously dormant latent bacilli at a site of dissemination.
DIAGNOSIS
The diagnosis of tuberculosis disease in adults is mainly bacteriologic, but in children, it usually is epidemiologic and indirect. In the absence of a positive culture result, the strongest evidence for tuberculosis in a child is history of exposure to an adult with contagious disease. The importance of an adequate history and exposure tracings cannot be overemphasized. Less direct methods, such as the tuberculin skin test and other laboratory tests, offer supportive information.
Tuberculin Skin Test
A positive tuberculin skin test result is the hallmark of tuberculosis infection. Within 8 years of his discovery of the tubercle bacilli, Robert Koch found that subcutaneous injection of a broth culture filtrate of tubercle bacilli, which he called tuberculin, produced fever, chills, vomiting, and induration at the injection site in a person with tuberculosis. The diagnostic usefulness of this test was described in the early twentieth century. In 1934, Florence Seibert developed a purified protein derivative (PPD) from broth cultures, which became the standard known as PPD-S.
The gold standard tuberculin test is the Mantoux, an intradermal injection of 5 tuberculin units (TU) of PPD in 0.1 mL of diluent containing the stabilizing agent polysorbate 80. This test is standardized and quantitative; results are interpreted as the transverse diameter of induration at 48 to 72 hours. The Mantoux test is subject to a variety of influences related to the test procedure (Table 179.2). The testing technique must be precise and consistent. Although experienced health care providers demonstrate good intraobserver agreement in interpretation, inexperienced observers, especially parents, often report results inaccurately.
A variety of host-related factors, such as age, nutrition, immunosuppression, viral infections or immunization with live viral vaccines, administration of corticosteroids, and the presence of overwhelming tuberculosis, can depress tuberculin
reactivity. Approximately 10% of adults and children with culture-documented tuberculosis do not react initially to PPD; delayed hypersensitivity often appears after appropriate treatment is started. In adults, initial anergy to tuberculin is related to a poor prognosis, but it does not appear to be true for children. A negative skin test result never rules out tuberculosis.
reactivity. Approximately 10% of adults and children with culture-documented tuberculosis do not react initially to PPD; delayed hypersensitivity often appears after appropriate treatment is started. In adults, initial anergy to tuberculin is related to a poor prognosis, but it does not appear to be true for children. A negative skin test result never rules out tuberculosis.
TABLE 179.2. FACTORS THAT CAN INFLUENCE MANTOUX TEST RESULTS | |
|---|---|
|
Recent exposure to environmental nontuberculous mycobacteria (NTM) can result in cross-sensitization and a false-positive reaction to PPD. This problem is especially common in the southeastern United States, where NTM occur in the environment, especially the soil. Cross-sensitization with NTM usually causes a reaction of less than 10 mm to a 5-TU Mantoux test, although reactions up to 15 mm can occur. This cross-sensitization tends to wane over a period of months. Prior immunization with bacille Calmette-Guérin (BCG) also may cause a significant Mantoux reaction, which often is smaller than 10 to 12 mm and usually wanes within 3 to 5 years. Because the effect of BCG on the skin test is variable and a reaction of 10 mm or more in a previously BCG-vaccinated child usually indicates infection with M. tuberculosis, the interpretation of the skin test result should be the same for a BCG-vaccinated child as it would be for a comparable, nonvaccinated child. Prior receipt of BCG vaccine is not a contraindication to receiving a tuberculin skin test.
The important issue for interpreting the Mantoux test result is the amount of induration that should be considered as likely to indicate tuberculosis infection. This amount varies with the population tested and depends on epidemiologic factors. Some overlap in reaction to the Mantoux test between groups of people with and without tuberculosis infection always occurs. False-positive and false-negative results always will occur, no matter what amount of induration is selected. Because of the critical contribution of epidemiology to the interpretation of the skin test, the size of induration considered positive should vary for groups according to their risk for acquiring tuberculosis infection. For adults and children at the highest risk of having tuberculosis infection progress to disease—those who are recent contacts of adults with infectious tuberculosis, who have abnormalities on chest radiography or clinical evidence of tuberculosis, or who are infected with HIV or have other causes of immune compromise—induration of 5 mm or more is classified as positive, indicating likely infection with M. tuberculosis. For other high-risk groups (see Table 179.1), including all infants, and for children living with adults in high-risk groups, induration of 10 mm or more is a positive result. Although raising the amount of induration considered positive to 15 mm for children at low risk for acquiring tuberculosis may be a scientifically sound practice in some locales, this strategy presents some practical problems, the most important of which is the difficulty of establishing that a child truly has no risk factors for acquiring tuberculosis infection. The American Academy of Pediatrics and Centers for Disease Control and Prevention recommend that children from low-prevalence populations with no specific risk factors should not be tested routinely, but if they are tested, 15-mm or larger indurations should be considered positive.
Laboratory Tests
Routine laboratory tests, such as a complete blood count and differential, erythrocyte sedimentation rate, and urinalysis, rarely aid in establishing the diagnosis of tuberculosis. Abnormalities of serum liver enzyme tests may be helpful in diagnosing miliary tuberculosis. Analyses of infected body fluids (e.g., pleural, joint, cerebrospinal) demonstrating lymphocytes, elevated protein, and decreased glucose suggest tuberculosis. These fluids and sputum should be examined microscopically with acid-fast stain to detect mycobacteria.
The most important laboratory test for establishing the diagnosis and managing tuberculosis is the mycobacteria culture. In adults, isolation of the organism confirms the diagnosis, and susceptibility test results direct therapy. Isolation of M. tuberculosis is not essential to the diagnosis of tuberculosis in children if epidemiologic, skin test, clinical, and radiographic findings are compatible with the disease. If culture and susceptibility tests are available from the adult source case, cultures from the child add little to management. However, when the source case is unknown, especially in areas with high rates of drug resistance, attempts should be made to isolate the organism from the child. Cultures should be obtained in any child with suspected extrapulmonary tuberculosis to confirm the diagnosis. Sputum produced by an older child or adolescent with pulmonary tuberculosis often yields M. tuberculosis. Younger children rarely produce sputum spontaneously, but it may be induced by use of hypertonic saline aerosol treatment. Gastric aspirates yield the organism in 30% to 40% of children; the yield is even greater in infants. When obtained correctly, gastric aspirates have a greater yield than do samples from bronchioalveolar lavage. Aspiration should be done early in the morning, as the child awakens, before the overnight accumulation of secretions swallowed from the respiratory tract is emptied from the stomach. The aspirates should be collected using saline-free fluid, and the pH should be neutralized if processing will be delayed for more than several hours.
Traditional culture methods using classic media such as Löwenstein-Jensen or simple synthetic media such as Middlebrook 7H10 require 4 to 6 weeks for isolation of the organism and another 2 to 4 weeks for susceptibility testing. The radiometric system uses liquid media containing fatty acid substrates labeled with carbon-14. As the mycobacteria metabolize the fatty acids, carbon dioxide-14 is released and measured as a marker of bacterial growth. A second substrate is used to differentiate NTM from M. tuberculosis. This system yields culture and susceptibility results within 7 to 10 days and is more sensitive than are traditional media for sputum cultures.
Many laboratories now use DNA probes to identify and speciate mycobacteria after they have been isolated in media. These probes use DNA sequences that are complementary to specific RNA or DNA sequences of M. tuberculosis. The sensitivity and specificity of these probes when used on isolated organisms approach 100%. Unfortunately, the sensitivity decreases precipitously when these probes are used directly on patient samples.
The technique of nucleic acid amplification (NAA) markedly increases the sensitivity of DNA testing directly on patient samples. The target DNA is isolated, replicated thousands of times using DNA polymerase and thermal cycling, and then detected using a nucleic acid probe or specially stained electrophoresis gels. The sensitivity and specificity of NAA for M. tuberculosis on sputum samples from adults with pulmonary tuberculosis that test positive with acid-fast stain have been greater than 95%, and the results can be available in less than 48 hours. Unfortunately, the various NAA techniques are approved for use only on samples that have positive results from the acid-fast stain because the specificity of the test is too low for routine testing of stain-negative samples. Several studies of the use of NAA on gastric aspirate samples from children with pulmonary tuberculosis have demonstrated a sensitivity slightly greater than culture (approximately 50%), but false-positive results also occur. The NAA for M. tuberculosis may be especially valuable to evaluate children with HIV infection who may have pulmonary tuberculosis. The various techniques are being adapted for use on other samples and tissues to aid in the diagnosis of extrapulmonary tuberculosis.
The technique of nucleic acid amplification (NAA) markedly increases the sensitivity of DNA testing directly on patient samples. The target DNA is isolated, replicated thousands of times using DNA polymerase and thermal cycling, and then detected using a nucleic acid probe or specially stained electrophoresis gels. The sensitivity and specificity of NAA for M. tuberculosis on sputum samples from adults with pulmonary tuberculosis that test positive with acid-fast stain have been greater than 95%, and the results can be available in less than 48 hours. Unfortunately, the various NAA techniques are approved for use only on samples that have positive results from the acid-fast stain because the specificity of the test is too low for routine testing of stain-negative samples. Several studies of the use of NAA on gastric aspirate samples from children with pulmonary tuberculosis have demonstrated a sensitivity slightly greater than culture (approximately 50%), but false-positive results also occur. The NAA for M. tuberculosis may be especially valuable to evaluate children with HIV infection who may have pulmonary tuberculosis. The various techniques are being adapted for use on other samples and tissues to aid in the diagnosis of extrapulmonary tuberculosis.
Diagnostic Criteria
The diagnosis of tuberculosis disease in children often is based on epidemiologic, clinical, radiographic, and skin test information, rather than mycobacteriologic data. The diagnosis of tuberculosis disease is confirmed if M. tuberculosis is isolated from any body site or if the clinical, radiographic, or histologic findings are consistent with tuberculosis and at least two of the following criteria are met: (a) a 5-TU Mantoux test yields more than 5 mm of induration, (b) other disease entities are ruled out and the subsequent clinical course and response to therapy are consistent with tuberculosis, and (c) an adult source case with contagious tuberculosis is discovered. Various clinical scoring systems, which assign points to various signs and symptoms, have been developed, but none has been subjected to clinical trials and most are considered to have limited usefulness.
CLINICAL MANIFESTATIONS
Endothoracic Disease
Asymptomatic Primary Tuberculosis
Asymptomatic, primary tuberculosis is an infection associated with tuberculin skin reactivity in the absence of clinical or significant radiographic findings. This type of infection is seen more commonly in school-aged children than in young infants; 80% to 95% of infected older children have clinically silent tuberculosis infections, but only 50% to 60% of infected infants remain asymptomatic. These children usually are treated with a single antituberculosis drug. Contact tracing to determine the origin of the infection is important, especially for infants and young children who were infected recently.
Primary Pulmonary Tuberculosis
The initial pulmonary complex includes the parenchymal focus and regional lymphadenitis. Approximately 70% of primary foci are subpleural, and localized pleurisy is a common component of the primary complex. The primary infection begins with the deposition of infected droplets into lung alveoli. The initial parenchymal inflammation usually is not visible on chest radiography, but a localized, nonspecific infiltrate may be seen. All segments of the lung are at equal risk of being seeded. In 25% of cases, multiple primary foci are present in the lungs. The infection spreads early to regional (usually hilar or mediastinal) lymph nodes. If tuberculin hypersensitivity develops within 3 to 10 weeks after infection occurs, the inflammatory reaction in the lung parenchyma and lymph nodes intensifies. The hallmark of primary tuberculosis in the lung is the relatively large size and importance of the adenitis compared with the relatively small size of the initial parenchymal focus. Because of the patterns of lymphatic drainage, a left-sided parenchymal focus often leads to bilateral hilar adenopathy, but a right-sided focus is associated with right-sided adenitis only.
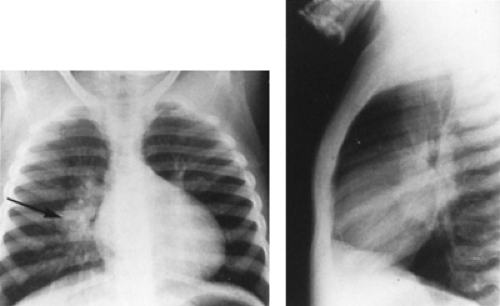
In most cases, the parenchymal infiltrate and adenitis resolve early. In some children, especially infants, the hilar lymph nodes continue to enlarge (Fig. 179.1). Bronchial obstruction begins as the nodes compress the associated regional bronchus. Inflammation intensifies, and the nodes may erode through the bronchial wall, leading to formation of thick caseum in the bronchial lumen and eventual occlusion of the bronchus. The common sequence for the development of endobronchial disease is hilar adenopathy, localized emphysema (caused by partial obstruction), and then atelectasis. The resulting radiographic shadows have been called collapse-consolidation or segmental tuberculosis (Fig. 179.2). The radiographic findings are similar to those seen with foreign body aspiration. Segmental lesions are seen most commonly in infants because of the small diameters of their bronchi. These lesions tend to occur within 6 months of the development of initial infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree