Tricuspid Atresia
David J. Driscoll
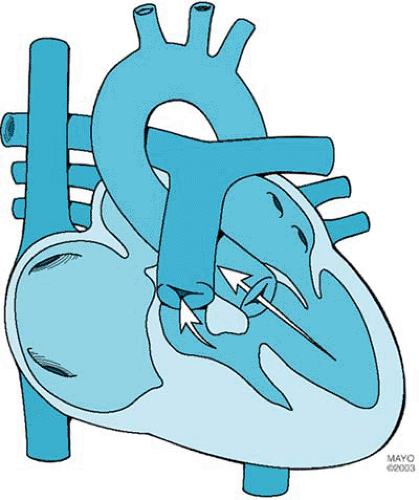
Tricuspid atresia, the third most common form of cyanotic congenital heart disease, consists of complete agenesis of the tricuspid valve and absence of direct communication between the right atrium and the right ventricle (Fig. 264.1). The prevalence of tricuspid atresia in clinical series of patients with congenital heart disease ranges from 0.3% to 3.7%. The prevalence rate in autopsy series is 2.9%. Tricuspid atresia occurs in one in 10,000 to one in 17,857 live births.
PATHOLOGY
Tricuspid atresia is divided into three types on the basis of the great artery relationship: I, normally related great arteries; II, D-transposed great arteries; and III, L-transposed great arteries (Table 264.1). The three types are subclassified according to the presence or absence and size of the ventricular septal defect and the presence or absence of pulmonary atresia or stenosis. Approximately 70% of cases are type I, 23% are type II, and 7% are type III.
An opening in the atrial septum allows egress of blood from the right atrium. The interatrial communication can be (or can become) restrictive. Additional cardiovascular abnormalities occur in 18% of patients with normally related great arteries and in 63% of patients with transposed great arteries. These abnormalities include coarctation of the aorta, patent ductus
arteriosus, and right aortic arch. Extracardiac anomalies occur in 20% of cases.
arteriosus, and right aortic arch. Extracardiac anomalies occur in 20% of cases.
TABLE 264.1. CLASSIFICATIONS OF TRICUSPID ATRESIA | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
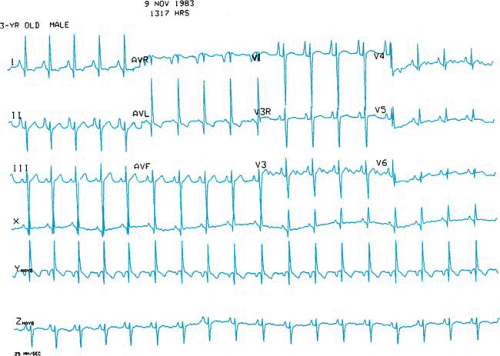
In tricuspid atresia, the left bundle of the cardiac conduction system originates early from the bundle of His and is unusually posterior and short. Presumably, this anatomic malformation of the conduction system accounts for the leftward or superior frontal plane axis of the electrocardiogram in patients with tricuspid atresia (Fig. 264.2).
HEMODYNAMICS
In tricuspid atresia, hemodynamic features depend on the presence or absence of pulmonary atresia, the degree of pulmonary stenosis, the presence of normally related or transposed great arteries, and the presence or absence of subpulmonary or subaortic stenosis. Because all systemic venous return (blood oxygen saturation low) and pulmonary venous return (blood oxygen saturation high) mix in the left atrium, the level of blood oxygen saturation reaching the left ventricle (and subsequently the aorta) depends on the relative volumes of pulmonary venous return and systemic venous return. Patients with tricuspid atresia and increased pulmonary blood flow (i.e., no pulmonary or subpulmonary stenosis) have a volume of pulmonary venous return relatively larger than that of systemic venous return and have relatively high systemic arterial blood oxygen saturation. In contrast, patients with relatively low pulmonary blood flow (caused by pulmonary or subpulmonary stenosis or pulmonary atresia) may have marked systemic arterial hypoxemia. The volume of pulmonary blood flow and the clinical characteristics may change. Patients with pulmonary stenosis may depend on patency of the ductus arteriosus for pulmonary blood flow, and, as the ductus closes, pulmonary blood flow may decrease significantly.
In general, pulmonary obstruction or subpulmonary obstruction (at the level of the ventricular septal defect [VSD]) is present or occurs during the first year of life in most patients with tricuspid atresia and normally related great arteries. In contrast, most patients with tricuspid atresia and D-transposed great arteries (type II-C) have unobstructed pulmonary blood flow. Hence, patients in the former groups usually have more marked hypoxemia than do those with D-transposed great arteries; however, those with D-transposed great arteries are more likely to have pulmonary edema and congestive heart failure and to develop pulmonary vascular obstructive disease. The ventricular septal defect associated with tricuspid atresia tends to become smaller. A restrictive ventricular septal defect (one in which the area of the VSD is smaller than the area of the aortic valve annulus) associated with normally related great arteries produces progressive subpulmonary stenosis and increasing hypoxemia. A restrictive ventricular septal defect associated with transposed great arteries produces subaortic obstruction. When combined with pulmonary stenosis or in the presence of a pulmonary artery band, significant left ventricular hypertension and hypertrophy occur.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree